It has been odd right from the start. If SARS-CoV-2 (the novel coronavirus that causes Covid-19) uses the angiotensin-converting enzyme 2 (ACE2) as a receptor to infect cells, then why is it a lung disease when the lungs have low levels of ACE2?This was determined back in May 2020 in a study finding that alveolar cell type 2 (the primary target of SARS-CoV-2) in the lungs has ACE2 expression that is "4.7-fold lower than the average expression level of all ACE2 expressing cell types," such as the liver, stomach, ileum, kidney, and colon.
Later, a systematic review published over a month ago further points out that SARS-CoV-2 tend to end up in places not consistent with ACE2 expression levels. Looking at the figure below, organs SARS-CoV-2 tend to localize (e.g., lungs and brain) do not always have high expression of ACE2, and vice-versa.
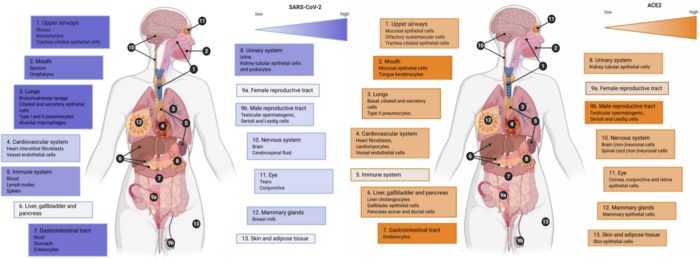
Right: Gradient color (orange) indicates low to high evidence for ACE2 expression; the highest expression was detected in the oral cavity, gastrointestinal tract, and the male reproductive system.
Neuropilin-1
In 2009, a study discovered that neuropilin-1 could bind to genes with patterns conforming to the C-end rule. Apparently, the genetic sequence of SARS-CoV-2 spike protein also has this C-end rule.
So, two independent groups of international researchers sought to test if SARS-CoV-2 could use neuropilin-1 as a receptor. The resulting two papers were published last month in Science with the titles, "Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity," and "Neuropilin-1 is a host factor for SARS-CoV-2 infection." Judging from the titles alone, we know the hypothesis is confirmed to be true.
In short, they showed that SARS-CoV-2 can still infect cells deficient in ACE2, as long as they express neuropilin-1. If both ACE2 and neuropilin-1 receptors are present, the infection became more infectious. Using genetic editing to cut or antibodies to block neuropilin-1, in turn, prevent SARS-CoV-2 infection.
"Whereas ACE2 was detected at very low levels, [neuropilin-1] were abundantly expressed in almost all pulmonary and olfactory cells, with the highest levels of expression in endothelial cells."Binding to neuropilin-1 would hinder its normal function, which is to aid neurons to make connections and blood vessels to grow. This explains why SARS-CoV-2 can attack the nervous and vascular systems. This explains why Covid-19 is also a neurological and vascular disease. And why SARS-CoV-2/Covid-19 attacks the lungs and sense of smell since neuropilin-1 is highly expressed in the respiratory and olfactory tracks.
As one of the studies in Science stated:
"Whereas ACE2 was detected at very low levels, [neuropilin-1] were abundantly expressed in almost all pulmonary and olfactory cells, with the highest levels of expression in endothelial cells."Endothelial means blood vessels; thus, neuropilin-1 concentrates in blood vessels and cells of the lungs and nose. This study also did an autopsy of olfactory cells isolated from the nose of deceased Covid-19 victims, showing that cells positive for neuropilin-1 — but lacking ACE2 — were still infested with SARS-CoV-2 particles.
Interestingly, another research group had found that rats with chronic neuropathic pain no longer feel pain when the spike protein of SARS-CoV-2 blocked neuropilin-1. This is because neuropilin-1 participates in pain excitation in neurons. In an anecdote, a 49-year-old South African male suffered a nerve injury in a motor accident in 2011. As a result, he lived with chronic pain — so unbearable that it disrupts his sleep every night — until he got Covid-19.
"I found it very strange: When I was sick with Covid, the pain was bearable. At some points, it felt like the pain was gone. I just couldn't believe it," he said. "I lived a better life when I was sick because the pain was gone." But the man recovered from Covid-19, and his pain resurfaced.CD147 (Basigin)
This month, a new study was published in the Nature-partnered journal, Signal Transduction and Targeted Therapy, titled "CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells." Again, the title has said it all. Researchers in China discovered that SARS-CoV-2 could infect human cells and mice expressing CD147 (also called basigin). SARS-CoV-2 infection can be further blocked with Meplazumab (an anti-CD147 antibody) or boosted with increasing CD147 expression.
"These findings demonstrate that CD147 provides more entries for SARS-CoV-2 infection and plays a potential role in mediating virus infection, especially in ACE2-deficient cell types."More importantly, this study showed that T-cells (both CD4+ and CD8+) collected from human blood were susceptible to SARS-CoV-2 infection via CD147. Notably, the study also confirmed that these T-cells do not express the ACE2 receptor, which is expected as T-cells are inherently ACE2 deficient.
The T-cell's CD147 functions as a receptor to receive chemotaxis signals. Chemotaxis is the attraction or recruitment of immune cells to the area where foreign invaders are located. Thusly, CD147 expression heightens when T-cells are activated. As follows, in the above study, activated T-cells were also shown to be more susceptible to SARS-CoV-2 infection than inactive T-cells. This also explains why Covid-19 tends to reduce T-cell count in severe cases.
In addition, the study determines the levels of ACE2 and CD147 expressions in human lung cells — BEAS-2B, and Vero E6 bronchial cells. Apparently, CD147 expression was 2.3-fold higher than ACE2 in Vero E6 cells, and ACE2 wasn't even expressed in BEAS-2B cells.
"These findings demonstrate that CD147 provides more entries for SARS-CoV-2 infection and plays a potential role in mediating virus infection, especially in ACE2-deficient cell types," the study authors wrote.Exploiting the CD147 receptor is not unique to SARS-CoV-2. It's also a receptor that the human immunodeficient virus type 1 (HIV-1) — that causes AIDS — uses. As T-cells are important for the specialized killing of infection, it's not unexpected that viruses evolve ways to counter T-cells.
Short abstract
We thought ACE2 is the only receptor SARS-CoV-2 can use for almost a year, which is odd given that the lungs don't even have a high ACE2 expression. But in the past month, two additional receptors of SARS-CoV-2 were discovered. One is neuropilin-1, which is highly expressed in the respiratory and olfactory tracts, specifically in cell types that SARS-CoV-2 preferentially infects. The second is CD147 (also called basigin), which is highly expressed in T-cells and bronchial cells in the lungs. This clarifies why Covid-19 tends to kill certain lung cells and T-cells that don't have ACE2.
Overall, these two newly identified receptors of SARS-CoV-2 explain many facets of Covid-19 that ACE2 alone cannot. We now know why Covid-19 is a mix of pulmonary, vascular, neurological, immunological, and olfactory diseases.



We think nuclear engineering is great.
When you can control and inhabit the nucleus, you control everything.
And this is how life improves.
And this is how we win the war against ignorance and put a stop to it.
When you really know your stuff.
Like we do!
signed,
laboratory and highly technical, trained scientists (and their agencies) concerned for life
(sarcasm)