
The package arrived in a plain cardboard box. It was simply addressed to S Neumann & Co - a mining sales agency in the centre of London - and weighed just over a pound (around 500g). But this was no ordinary cargo.
It was April 1905, and three months earlier, the surface manager at the Premier Mine in South Africa had been completing a routine inspection 18ft (5.4m) underground, when he glimpsed a reflected light in the rough wall above him. He assumed it was a large piece of glass hammered in by colleagues as a practical joke. Just in case, out came his pocket knife, and after some digging... the knife promptly snapped. Eventually the rock was removed successfully, and revealed to be a bona fide diamond - a monster 3,106.75-carat stone, almost the size of a fist. It was not only enormous, but unusually transparent.
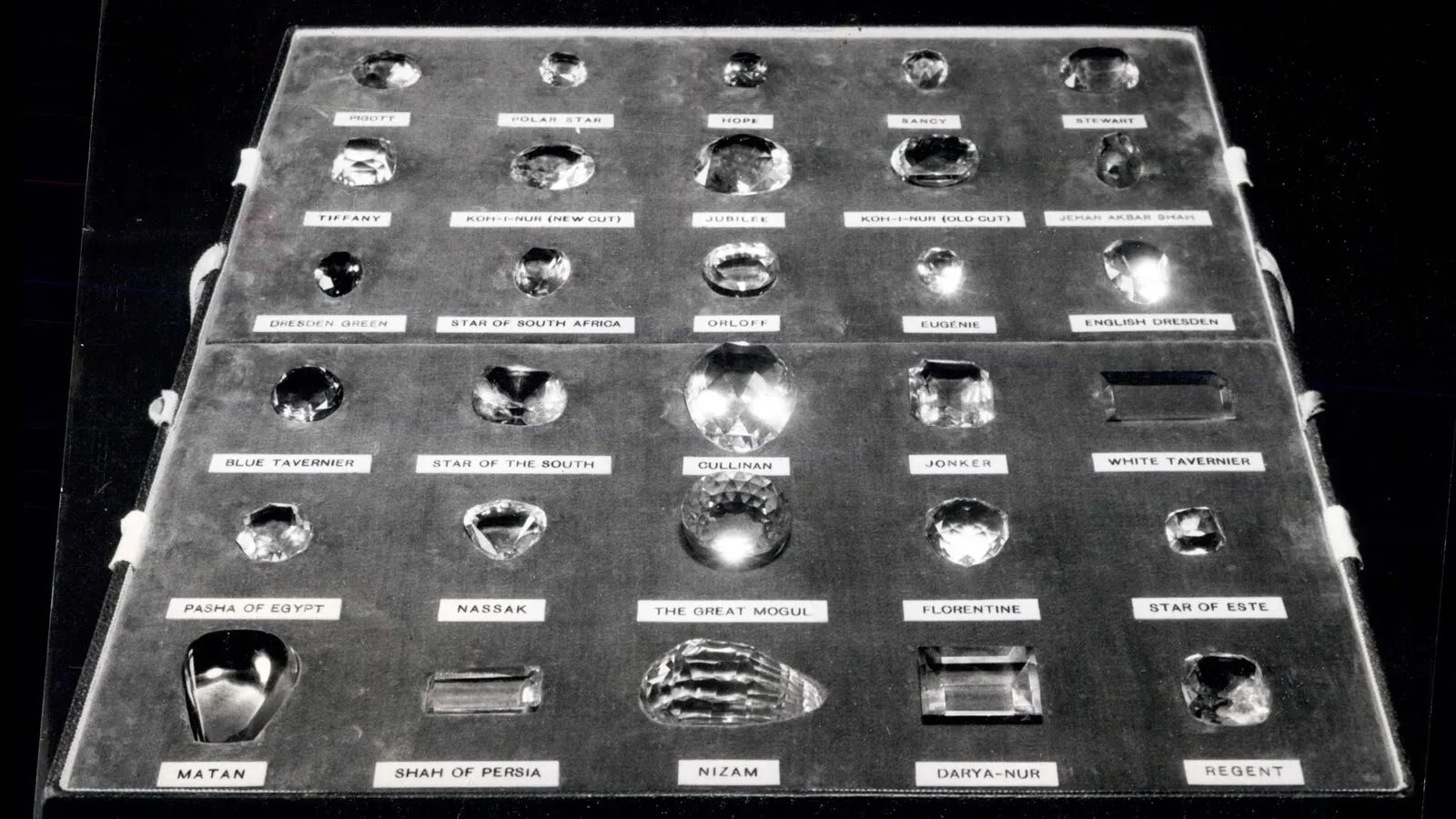
The Cullinan, as it became known, is the largest diamond ever found. Once it had been polished and cleaved into several more manageable stones, the largest crystal it yielded would shine like the cool glow of a star in a distant galaxy. As a result, this stone - the Cullinan I - is sometimes known as the Great Star of Africa.
Nearly 120 years later, the mega-diamond has not been forgotten. During the late Queen Elizabeth II's funeral procession, several of the Cullinan's descendants were placed on the Monarch's coffin, and rode along with her - they were only removed as she was lowered into the royal vault. That's because today these giant gems are part of the Crown Jewels, normally kept in the Tower of London and brought out for state events - the Cullinan I now resides in the British Sovereign's Sceptre, while its next-largest sibling, the Cullinan II, is embedded in the Imperial State Crown.
However, before the rough diamond could have its makeover and take its place in history, it needed to be sold - and London was chosen as the most promising location to do this. This presented a problem: how do you transport such a valuable stone 7,926 miles (12,755km), without it being stolen?

But though they're renowned across the globe for their size and transparency, these characteristics are no accident. The Cullinan was a "Clippir" diamond - a member of a special category of the very largest and clearest examples ever found. And there is more to them than meets the eye.
For all their beauty, diamonds are really fragments of the deep Earth - intriguing geological anomalies disguised as mere jewellery. These strange rocks are capsules from another world - a mysterious realm of unfathomable pressures, swirling green rock, and elusive minerals, far below the Earth's surface. Scientists around the globe have been studying them for decades to reveal the region's secrets - and intriguingly, it's the very diamonds that we value most that have the most unusual stories to tell. In fact, large rocks like the Cullinan are transforming our understanding of the inside of our planet.
An unusual opportunity
Sitting in front of a microscope at the Gemological Institute of America (GIA) in 2020, Evan Smith carefully stretched some rubber gloves over his fingers, and peered into the instrument's lenses. Beneath was a diamond worth almost as much as a small country - about the size of a walnut, with 124 carats of wonderous brilliance.
To reach this point, Smith had already navigated almost military levels of security - iris scans and identity checks, followed by layer after layer of locked doors, secure lifts and mysterious restricted corridors. While he worked, video cameras streamed a constant view of the room to watchful security guards.

Maya Kopylova, a professor of mineral exploration at the University of British Columbia, says getting samples of any diamonds is hard, and most of the diamonds she works with would have otherwise been thrown away. "Researchers have to have a good relationship with companies and they will never give you valuable samples," she says. "So, they will never give us diamonds that are 6mm (0.2 inches) in size or larger."
Even then, acquiring them is convoluted and expensive - first, Kopylova has to visit the high-security facilities where diamonds are sorted and identify the specimens she'd like to study. Once the acquisition has been approved, then comes the paperwork - all diamonds must travel with a Kimberley Process certificate, which proves its provenance and helps to prevent conflict or "blood" diamonds from entering the market.
However, Smith's situation is different. At GIA, he has access to one of the largest collections of diamonds on the planet - millions of gems that have been sent there to be valued, so that they can be insured or sold. "If you want to see something rare and unusual, this is the perfect place to go because there are diamonds coming through here all the time," says Smith. "Every few days, you might get to borrow a diamond for maybe a few hours, maybe a day or two and study it."
A few years earlier, this is exactly what Smith had done. Together with an international team of scientists, he casually requisitioned 53 of the largest, clearest and most expensive available - including some from the same mine as the Cullinan diamond - and took them back to his laboratory to view under a microscope.
What Smith found was revolutionary. Nearly three-quarters of the Clippir diamonds contained tiny pockets, or "inclusions" of metal that had avoided rusting - not something you'd find in ordinary ones - while the remaining 15 contained a kind of garnet which only forms within the Earth's mantle, the layer above its molten core.
Together, these inclusions provide chemical clues that the diamonds could only have formed no fewer than 360km (224 miles) and no more than 750km (466 miles) underfoot. In this Goldilocks zone, it's deep enough to explain the metal inclusions that hadn't been exposed to oxygen, which is abundant higher up, and it's not so deep that the garnet rocks would have broken down under the immense pressures of the lower mantle. Ordinary diamonds, meanwhile, originate below the crust, just 150-200km (93-124 miles) down.
For his 2020 study - together with Wuyi Wang, who is vice president of research & development at GIA - Smith analysed the 124-carat diamond and found that it formed at the deeper end of the possible range - at least 660km (410 miles) below the Earth's surface.

"From a geological perspective, diamonds [in general] are really strange minerals," says Smith. It just so happens that our species finds them so beguiling, we invest tens of millions every year in our quest for them - well beyond the budget of any research project.
And while these endeavours have led to much destruction, from wars and colonisation to the rerouting of rivers and upending of rare habitats, if it weren't for our enthusiasm for these sparkling lumps of carbon, "we would have no idea about this story [of their unusual properties], because we would never get to recover them and study them", says Smith.
Even the most ordinary diamonds are unique among rocks, forming far deeper than any others - there is nothing else at the Earth's surface that has emerged from further down into our planet. "There are no other materials at the surface coming from a depth of 600km [373 miles], absolutely not," says Kopylova. Magma that reaches us comes from around 400km [249 miles] down, but unlike diamonds, which reach the surface unchanged, this is melted rock. "And that adds another degree of uncertainty of what was the original material before it was affected by melting," adds Kopylova.
Every diamond that has ever been sold or worn, except those grown in the laboratory, is at least 990 million years old - formed at a time when strange, spaghetti-like lifeforms, primitive algae, were just beginning to creep onto land. But some are truly ancient, first crystallising at least 3.2 billion years ago, when the entire planet may have been one big ocean - a swirling blue orb with no visible land or continents whatsoever.
Once a diamond has formed, it takes a sequence of unlikely processes to bring it up to the surface. First, the natural movement of super-heated rock in the mantle brings it closer to the surface over hundreds of millions of years, possibly as part of giant "plumes" which can stretch thousands of kilometres from the edge of the core to the upper mantle.
Then the diamond has to be in the right place at the right time, to be blasted up in magma. "It [the molten liquid] has picked up those diamonds from a variety of different locations and kind of mixed them together," says Smith. This diamond-flecked magma then solidifies into rock within the Earth's crust - specifically one called kimberlite - where the gemstones may be discovered millions of years later.

The Cullinan diamonds
Once it had arrived in London safely, the rough Cullinan diamond was sent to be cut by Joseph Asscher. It's reported that the rock was so massive, the first heavy blow of the hammer led to yet another knife casualty (it broke) - and made him faint. However, eventually Asscher managed to divide the diamond into nine major stones, the largest of which was 530.20 carats - a measure of its weight - and 96 smaller ones. While the larger stones became part of the British Crown Jewels or the British monarch's private collection, the smaller fragments were sold to various clients around the world.
Around the same time, they noticed a puzzling pattern. Most diamonds - called Type I - contain a significant amount of nitrogen, which affects their crystal structure and can add a hint of pale yellow or brown. Occasionally, though, a diamond has almost no detectable traces of this element. These are the Type II diamonds, and the phenomenon is extremely rare - except in the very largest diamonds.
"It's not just that they're big that sets them apart," says Smith. "When you look at these big, high-quality [type II] diamonds, like the Cullinan, there turned out to be something strange about them, that makes them more likely to fall into this category that should otherwise be something very rare. So this was kind of a long-standing mystery."
Eventually scientists discovered that some diamonds are "super-deep", and identified a handful of mines where they were most likely to be found - including the Cullinan mine in South Africa and Letseng mine in the nearby kingdom of Lesotho, where Smith's 124-carat specimen originated.

The final clincher in Smith's 2020 study was an elusive mineral that was only seen for the first time six years earlier - found in a 4.5 billion-year-old meteorite that slammed into the Earth back in 1879.
It's thought that the ancient extra-terrestrial rock had once been part of a much larger celestial object, an asteroid, and broke off during a catastrophic impact. During this process, it experienced staggeringly high pressures, similar to those found within the Earth.

And in 2014, scientists glimpsed the mineral bridgmanite within one of these alien rocks. Though it's the most abundant material on Earth, it can only exist at the high pressures found in the lower mantle, the layer above the Earth's molten core. Like many high-pressure minerals, it breaks up when it gets to the surface - and this was the first time it had ever been seen.
Amazingly, the 124-carat gem Smith studied contained this very mineral, though in its broken-down form - even inside diamonds, it doesn't usually survive the journey up. This suggests that the glittering rock formed within the lower mantle, at pressures at least 240,000 times those at sea level. That's 240 times the crushing pressure in the deepest part of the ocean, the Mariana Trench.
But why does this all make super-deep diamonds so different? And what can they tell us about the hidden world they're made in?
Ancient carbon
According to Smith, the unusual qualities of the world's largest and most valuable diamonds are all down to the way they form.
Even the origins of regular diamonds are still somewhat mysterious, but they're thought to start life as a fluid - most likely ancient seawater trapped deep underground along with sinking oceanic plates. Somehow, perhaps due to a sudden change in temperature or pressure, this mineral-rich water ends up rejecting the carbon that's dissolved within it, which is precipitated out - and under the immense pressures below the Earth's crust, the carbon crystallises into diamonds.
But super-deep diamonds like the Cullinan are different. Instead of a beginning within water, these start life as carbon dissolved within liquid metal, far down in the planet's interior. "It's like molten iron nickel alloy with sulphur and carbon dissolved in that," says Smith. "So it's a totally different kind of fluid, but it's still carbon fluid. It's undergoing whatever chemical or temperature changes, and that's causing carbon to crystallise out." In this case, the initial fluid contains less nitrogen, so they end up with very little of this element - and are consequently more transparent.
In short, Clippir diamonds aren't just regular ones that have somehow grown to remarkable proportions - they're fundamentally different. In fact, their unparalleled size and transparency are a direct result of the unusual way they form. And since their discovery, super-deep diamonds have revealed some of our planet's most closely guarded secrets.
"I think the biggest thing they [super-deep diamonds] inform us about is the process of subduction - when an oceanic tectonic plate, sinks down into the Earth," says Smith.
This is the phenomenon we all learn about in classes at school - the Earth is split up into seven tectonic plates, which "float" around on the surface, generating earthquakes when they rub against one another, and volcanoes when they move apart or get too close. Crucially, while new plates are constantly being formed, some are also slowly slipping below the crust, never to be seen again.
But though scientists have long suspected that these vanished, subducting plates - which are usually heavier, oceanic ones - eventually drift down into the lower mantle, this has never been confirmed. "You can go to a volcano and say, 'yeah, this magma comes out of the Earth', or go to spreading centres of the oceans and see, that there's new crust forming...But it's really difficult to do the opposite and say, what's going down into the Earth?" says Smith.
Super-deep diamonds can provide important clues, because amazingly, these disappeared tectonic plates might be what they are made of. "So we've seen diamonds that look like they're essentially pieces of the oceanic crust that have been carried down to the lower mantle," says Smith. "These diamonds are physically telling us that this process is physically true."
Other than confirming what happens to oceanic plates that end up in the interior of our planet, super-deep diamonds also tell us about the kinds of things you might find in the lower mantle. For a start, there must be carbon, or the diamonds wouldn't exist. But in 2021, the discovery of a rare super-deep diamond from Juína, Brazil, hinted that there may also be whole "oceans" of water.
The diamond contains a pocket of a vivid blue mineral, hydrous ringwoodite, which is a high-pressure form of olivine, the green mineral that makes up most of the upper mantle. Under the microscope, it looks like a tiny shard of indigo glass - and this type contains around 2.5% water.
For years, scientists have believed that all the water at the Earth's surface - in rivers, ice sheets, lakes and oceans - ultimately comes from the mantle. But where exactly it could be stored has been up for debate, particularly because olivine does not store water well. However, the discovery of water-saturated ringwoodite suggests that it's stored lower down, in the same region where many super-deep diamonds form.
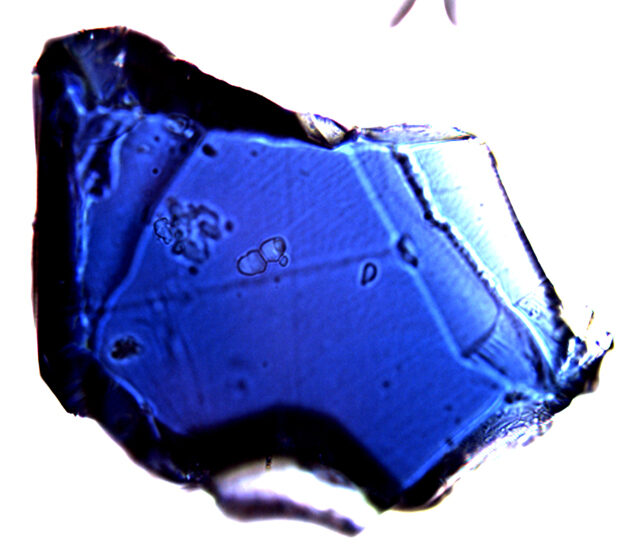
"There is definitely a wow factor when you're trying to scrutinise something under the microscope, but then you also have in the back of your mind this idea that the object you're handling is worth millions of dollars," says Smith. "And it struck me a few times, I mean, just looking at some of these things, and thinking about, 'Oh wouldn't it be great if we could break this open or study in more detail just because it's such a fascinating scientific sample'... but then you can't because it's such a valuable gemstone. There's kind of a weird duality."
Since smashing diamonds is generally frowned upon, Smith can't help longing for a less destructive - though no less radical - alternative: leaving diamonds in their rough form. When the rocks emerge from the Earth, they're lumpy and coarse, with none of the sparkle they acquire after they've been cut and polished - but the surface you see reads like a history of their adventures underground.
"The diamond can be chemically etched away by magma, and you end up with these really unusual shapes and intricate features... the natural surfaces that have been sculpted by all these different forces over millions of years. That is unique, and I see a lot of beauty in that."
Zaria Gorvett is a senior journalist for BBC Future and tweets @ZariaGorvett



Also that bit about British royalty being so in love with bling that Queen Elizabeth was adorned with them even in death. I suppose it is normal for most people upon their passing to be surrounded by the things they loved most in this life.