We thought only fools messed with the cast-iron laws of thermodynamics - but quantum trickery is rewriting the rulebook, says physicist Vlatko Vedra
A FEW years ago, I had an idea that may sound a little crazy: I thought I could see a way to build an engine that works harder than the laws of physics allow.
You would be within your rights to baulk at this proposition. After all, the efficiency of engines is governed by thermodynamics, the most solid pillar of physics. This is one set of natural laws you don't mess with.
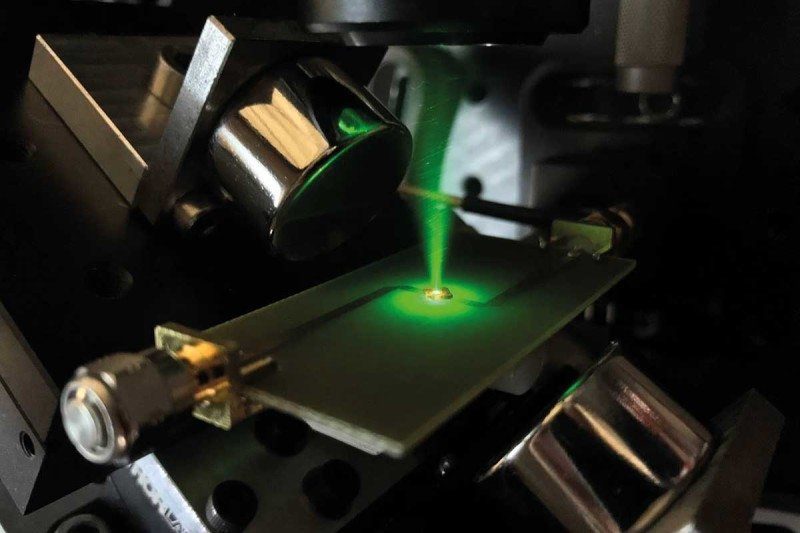
Yet if I leave my office at the University of Oxford and stroll down the corridor, I can now see an engine that pays no heed to these laws. It is a machine of considerable power and intricacy, with green lasers and ions instead of oil and pistons. There is a long road ahead, but I believe contraptions like this one will shape the future of technology.
Better, more efficient computers would be just the start. The engine is also a harbinger of a new era in science. To build it, we have had to uncover a field called quantum thermodynamics, one set to retune our ideas about why life, the universe - everything, in fact - are the way they are.
Thermodynamics is the theory that describes the interplay between temperature, heat, energy and work. As such, it touches on pretty much everything, from your brain to your muscles, car engines to kitchen blenders, stars to quasars. It provides a base from which we can work out what sorts of things do and don't happen in the universe. If you eat a burger, you must burn off the calories - or get fatter. Coffee never spontaneously warms up when set on a table. As the universe expands, it cools, heading unwaveringly towards heat death in the distant future. All these unavoidable truths spring from thermodynamics. In fact, they come from its two main laws, uncreatively named the first and the second laws.
These laws go back a long way, and one of my favourite episodes relating to their creation involves Julius von Mayer, a German doctor whose real passion was physics. The story goes that in the 1840s, Mayer got a job as a ship's surgeon on a voyage to Jakarta. During this, he noticed something curious: near the tropics, the blood in the sailors' veins wasn't blue as it would be back home in Germany, but deep red.
He hypothesised (wrongly, as it turns out) that the redder blood was due to less food being used to keep the body warm in the hotter climate. But in thinking about the give and take between metabolism, temperature and heat generation in the body, Mayer had alighted on the essence of the first law: energy can't be created or destroyed, merely passed around.
What came to be called the second law had its genesis about 20 years before Mayer boarded his ship. At this time, steam engines were transforming Europe, their furnaces and pistons driving the factories and mills of the industrial revolution. Sadi Carnot, a French engineer, was dissatisfied that no one had a rigorous understanding of how these engines worked, and set out to develop one.
His crucial insight was that, left to their own devices, hot things always spread warmth to their surroundings. When water is heated in steam engines, for example, some of the heat always leaks away to the air outside, so they are never perfectly efficient. In 1824, he published his only book generalising the idea to show that no engine can exceed a certain limit, now known as the Carnot efficiency. This depends on the temperature difference between the heat source (say, a fire) and the heat sink (say, the outside air).
Inescapable entropy
Carnot died a few years later, and his book was ignored for decades until German physicist Rudolf Clausius took notice. Carnot had conceived of heat as a weightless substance called caloric, but Clausius knew it was actually related to how fast atoms or molecules move. That enabled him to reformulate Carnot's ideas in terms of a measure of disorder he called entropy. Imagine you have a hot box of particles that are moving quickly and a cold box of slow-moving ones. That is an orderly arrangement because all the particles with similar energies are together. But the universe doesn't like low entropy states, said Clausius. If you open the boxes, the particles mix. This led him to the second law as we know it: entropy naturally increases unless you put in some work to stop it.
Follow the logic of the two laws and you end up with a cast-iron description of what's possible in the universe. The astrophysicist Arthur Eddington once said: "If your theory is found to be against the second law of thermodynamics I can give you no hope; there is nothing for it but to collapse in deepest humiliation."
Thermodynamics predates quantum theory; in fact, it was responsible for its birth. In 1900, the German physicist Max Planck was trying to understand the properties of a hypothetical object called a black body that absorbs all radiation falling on it and then emits it again. The best physics of the time suggested there were an infinite number of wavelengths, so the body would emit an infinite amount of energy. That was nonsensical. Planck solved the problem by supposing that energy can only come in chunks. He called them quanta.
That leap helped explain many niggling questions in physics. But when we began studying objects that perform according to the quantum playbook, we found they do extraordinary things. One of the best-known examples is entanglement, when two particles become intertwined so that interfering with one instantly changes the properties of the other. Another example is that an atom can simultaneously exist in a low and high-energy state, known as a superposition.
These behaviours break all the usual rules of dynamics. Is there any reason to think thermodynamics is exempt? Only in the past five years or so have we had the tools to probe this question. Take the work of Tobias Schaetz at the Freiburg Institute for Advanced Studies, Germany. In 2016, he described an experiment looking at ions inside a crystal. He gave them some energy and watched how they cooled. Unlike a cup of coffee, which cools gradually, the ions seemed to lose energy for a while, but then the energy suddenly bounced back. It is proof of what we had suspected: the rules of classical thermodynamics don't always apply in the quantum world.
Unfortunately, it is tricky to pin down what laws do apply. This is because there are no obvious quantum equivalents of classical thermodynamic concepts like heat or entropy. They are the ultimate product of the motions of many particles; so how do you begin to think of analogues when you are dealing with just one or two particles?
Well, never mind. I thought I would make a quantum version of a heat engine anyway. It is rather a different engine from anything Carnot would have been familiar with, but the principles are the same. The idea was to set up pairs of organic molecules and raise them to a high energy level by shining light on them. Left alone, the molecules will return to a slightly lower energy level, re-emitting light of a different frequency as they do so.
Here's the important part. If we set up the experiment just right, the emitted light won't carry any information that could tell us which of the two molecules it came from. According to quantum theory, this forces them to become entangled, so that when one drops to the lower energy level, the other one automatically does too, with both emitting light in unison in a process called superradiance. I expected that this quantum engine would still be subject to energy leakages in the manner Carnot identified nearly 200 years ago. But because of the superradiance, it should transfer energy faster, making it more efficient than a non-quantum engine.
Working with my two experimentalist colleagues, Tristan Farrow and Robert Taylor, I completed a control experiment last year in which the molecules weren't entangled. But just as we were putting the finishing touches to the interesting version, we were scooped.
In October 2017, my Oxford colleague Ian Walmsley and his team described an experiment similar to the one we had envisaged. In this engine, it was not organic molecules doing the absorbing and emitting, but atoms trapped inside special cavities in a diamond. The atoms weren't entangled, but were in a superposition of a high and low-energy state. And sure enough, Walmsley and his team saw that light was produced quicker than the classical rules of thermodynamics predict.
It isn't yet entirely clear why this is so. And admittedly, the degree of violation is tiny and wouldn't be useful in practice. Nonetheless, it is crucial first proof that quantum heat engines can bend those cast-iron rules.
I expect this machine can be improved upon and I am excited about the future of quantum heat engines. The thing that first drew me into this game is my work on quantum computers. There is plenty of talk about these futuristic machines, which operate using quantum bits, or qubits, and should be able to crack all sorts of intractable calculations. But getting them to work involves cooling the hardware to extremely low temperatures, which demands vast amounts of energy.
Descendants of Walmsley's machine could help. After all, a heat engine converts heat into directed work, for example to move a steam engine's piston. If you reverse that, you can use directed work to pump heat away. The result is a quantum fridge. Gleb Maslennikov at the National University of Singapore and his colleagues are already experimenting with quantum fridges, with promising indications that they too might be more efficient than their classical counterparts.
It's not just quantum computers that could benefit. One major obstacle to further miniaturising normal circuits is that they would overheat if we tried to cram components any closer. Better refrigeration is exactly what we need.
If you think quantum fridges sound handy, allow me to introduce the quantum battery. A former student of mine, Felix Binder, now at Nanyang Technological University in Singapore, has shown that quantum batteries can charge more quickly than normal ones.
Instead of moving ions around, as traditional batteries do, these devices would have electronic bits akin to a computer bit that can be either charged or not. Under classical thermodynamics, the amount of energy used to charge the battery increases linearly with the number of bits. But Binder has shown that if we entangle the bits, the amount of energy needed for a full charge scales with the square root of their number. This means that a quantum battery with 1 million bits would be fully charged in the time it would take to charge a 1000-bit classical battery. Vittorio Pellegrini at the Italian Institute of Technology in Genoa is one researcher hoping to build such a super-battery within a few years.
The untidiest room
But we shouldn't think that quantum thermodynamics is only about creating gizmos. It also touches the most profound distinction there is: life and death. Living things constantly strive against the second law of thermodynamics, sucking in energy to maintain the order within their cells. Powering all this are our bodies' equivalent of heat engines: mitochondria. So here's an intriguing question: given that natural selection tends to encourage efficiency, has biology evolved quantum heat engines? There is a hot debate about whether any quantum effects are important in biology, but in my opinion it's not crazy to think that evolution would produce the most efficient engines possible.
Even the flow of time might be recast by quantum thermodynamics. No physical law provides a reason why any natural processes can't go backwards - except the second law of thermodynamics. Its insistence that entropy must increase leads many physicists to suspect that time somehow arises from entropy changes.
In classical terms, entropy makes intuitive sense. For example, classical thermodynamics says the universe must be at least as disordered as its parts are. This is like saying that the overall messiness of a house, perhaps quantified as the amount of energy needed to tidy it up, can't be less than the messiness of the untidiest room.
The picture would be radically different if the universe obeys the laws of quantum thermodynamics. True, we don't know exactly what these are yet. But we do know from the equations of quantum theory that the overall amount of disorder in the universe must remain constant. What's more, quantum uncertainty forbids us from gaining full information about the states of individual parts of the universe, meaning that some parts can be more disordered than the whole.
This could mean that if you look at the universe as a whole, entropy doesn't change and so there is no time. But look at small patches where entropy is changing and time starts ticking. Because things don't have to add up everywhere, all the time, it is even possible that the arrows of time flow in different directions in different parts of the universe.
It is only by carefully probing the quantum foundations of thermodynamics that we will discern whether any of this is an accurate picture of reality. That's why quantum heat engines are so interesting. I can't wait to put mine through its paces.



Key statements in the article are contradictory and internally inconsistent - specifically: "As the universe expands, it cools, heading unwaveringly towards heat death in the distant future.", and ". . . we do know from the equations of quantum theory that the overall amount of disorder in the universe must remain constant ."
Einsteins comment ("If you can't explain it to a six year old, you don't understand it yourself.”) certainly appears to apply to Mr. Vidral.