
On Sunday, in an appearance on ABC's This Week, Albert Bourla was asked when the country should expect the shots to be approved in kids between ages five and 11.
The New York-based firm, along with its German partner BioNTech, recently released data that it said showed the vaccine was safe and effective in a smaller doses in elementary schoolers.
'I think we are going to submit this data pretty soon,' Bourla told host George Stephanopoulos.
'It's a question of days, not weeks, and then it is up to FDA to be able to review the data and come to their conclusions and approve it or not.'
According to clinicaltrials.gov, Pfizer's study in younger children worked similarly to the way it did in older children and adults.
Comment: At what cost to their long-term health?
A total of 4,500 younger kids from ages six months to 11 years were enrolled at nearly 100 clinical trial sites in 26 U.S. states, Finland, Poland and Spain
About half of the ages five-to-11 group were given two doses 21 days apart and the other half were given placebo shots.
The team then tested the safety, tolerability and immune response generated by the vaccine by measuring antibody levels in the young subjects.
Pfizer said it had selected lower doses for COVID-19 vaccine trials in children than are given to teenagers and adults.
Those aged 12 and older receive two 30 microgram (μg) doses of the vaccine.
However, children between ages five and 11 were given 10 μg doses and kids from six months to four years old will receive three μg doses.
Bourla assured that Pfizer would be ready to ship these smaller doses across the country if the FDA authorizes the shot in younger children.
'If they approve it, we will be ready with our manufacturing to provide this new formulation of the vaccine,' he told This Week.
'Because the vaccine that the kids will receive...it's a different formulation. It's one-third of the dose we are giving to the rest of the population.'
Unlike the larger clinical trial conducted in adults, the pediatric trial did not measure efficacy by comparing the number of COVID-19 cases among the vaccine group to the number in the placebo group.
Instead, scientists looked at levels of neutralizing antibodies in young vaccine recipients and compared the levels to those seen in adults.
Comment: Why the change in parameters? Is it because the vaccine has NOT reduced coronavirus cases? A recent article from the BBC titled 'Covid-19 in Wales: A third of positive cases are unvaccinated' did its best to distort the fact that 2 thirds of the positive cases are vaccinated. See also:
- Passengers on first fully vaccinated North American cruise test positive for COVID
- Over 500 people admitted to hospital with coronavirus after getting vaccinated
- US data show rising 'breakthrough' infections among fully vaccinated
- Stunning data from Israel: 95% of severely ill Covid patients are VACCINATED
The companies expect data on how well the vaccine works in children between ages two and five and between six months and two years of age by the end of the year.
Recently, pediatric cases also increased from 71,726 per week at the beginning of August to more than 243,000 earlier this month, fueled by the Delta variant.
Comment: Fearmongering. How many were hospitalized and/or seriously ill and how many were false positives?
However, they now appear to be trending downward with 225,000 reported last week, according to the American Academy of Pediatrics.
There have also been 480 pediatric deaths since the start of the pandemic, indicating children make up less than 0.1 percent of all deaths.
Comment: How many died 'with coronavirus vs. 'of' coronavirus?
Currently, no evidence suggests the Delta variant is more dangerous in kids than previous strains of the virus.
Because of this low risk of severe illness, polls have shown that many parents are not inclined to vaccinate their children.
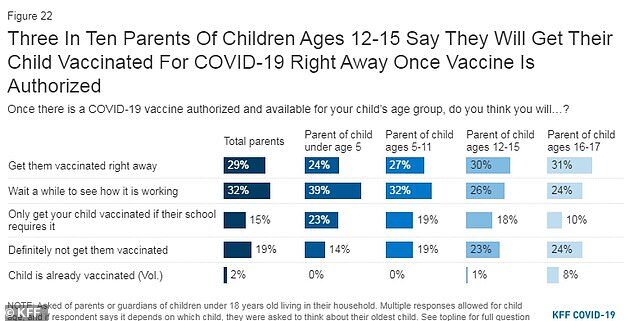
In an April 2021 poll, conducted by the Kaiser Family Foundation, parents were asked if they would get their child immunized once a COVID-19 vaccine is authorized and available for their child's age group.
Among parents of those between ages five and 11, 27 percent said they would get their child vaccinated 'right away' and 32 percent said they would wait and see how it's working.
Nineteen percent said they only plan to vaccinate their children if their school requires it and an additional 19 percent said their child will definitely not be getting vaccinated.
A July 2021 survey, conducted by CS Mott Children's Hospital National Poll on Children's Health at Michigan Medicine found similar results.
Among parents of children from ages three to 11, 49 percent said it was likely their kids would be getting a vaccine and 51 percent said it was unlikely.



[Link]