For all the ease with which the wildly popular CRISPR-Cas9 gene-editing tool alters genomes, it's still somewhat clunky and prone to errors and unintended effects. Now, a recently developed alternative offers greater control over genome edits — an advance that could be particularly important for developing gene therapies.
The alternative method, called prime editing, improves the chances that researchers will end up with only the edits they want, instead of a mix of changes that they can't predict. The tool, described in a study published on 21 October in Nature, also reduces the 'off-target' effects that are a key challenge for some applications of the standard CRISPR-Cas9 system. That could make prime-editing-based gene therapies safer for use in people.
The tool also seems capable of making a wider variety of edits, which might one day allow it to be used to treat the many genetic diseases that have so far stymied gene-editors. David Liu, a chemical biologist at the Broad Institute of MIT and Harvard in Cambridge, Massachusetts and lead study author, estimates that prime editing might help researchers tackle nearly 90% of the more than 75,000 disease-associated DNA variants listed in ClinVar, a public database developed by the US National Institutes of Health.
The specificity of the changes that this latest tool is capable of could also make it easier for researchers to develop models of disease in the laboratory, or to study the function of specific genes, says Liu.
"It's early days, but the initial results look fantastic," says Brittany Adamson, who studies DNA repair and gene editing at Princeton University in New Jersey. "You're going to see a lot of people using it."
Prime editing may not be able to make the very big DNA insertions or deletions that CRISPR-Cas9 is capable of — so it's unlikely to completely replace the well-established editing tool, says molecular biologist Erik Sontheimer at the University of Massachusetts Medical School in Worcester. That's because for prime editing, the change that a researcher wants to make is encoded on a strand of RNA. The longer that strand gets, the more likely it is to be damaged by enzymes in the cell.
"Different flavours of genome-editing platforms are still going to be needed for different types of edits," says Sontheimer.
But prime editing appears to be more precise and versatile than other CRISPR alternatives developed thus far. Those include modified versions of CRISPR-Cas9 that enable researchers to swap out one DNA letter for another, and older tools such as zinc-finger nucleases, which are difficult to tailor to each desired edit.
Freedom through control
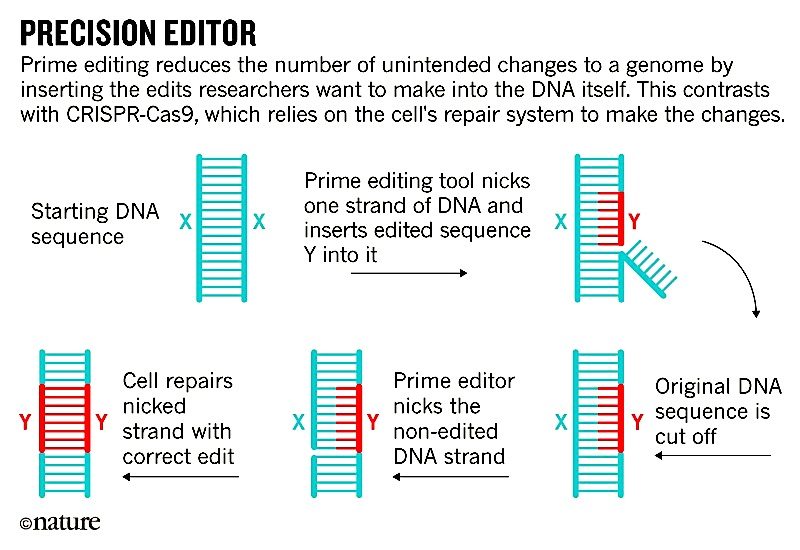
CRISPR-Cas9 and prime editing both work by cutting DNA at a specific point in the genome. CRISPR-Cas9 breaks both strands of the DNA double helix and then relies on the cell's own repair system to patch the damage and make the edits. But that repair system is unreliable and can insert or delete DNA letters at the points where the genome was cut. This can lead to an uncontrollable mixture of edits that vary between cells.
In addition, even when researchers include a template to guide how the genome is edited, the DNA repair system in most cells is far more likely to make those small, random insertions or deletions than to add a specific DNA sequence to the genome. That makes it difficult — and in some cases, nearly impossible — for researchers to use CRISPR-Cas9 to overwrite one piece of DNA with a sequence of their choosing.
Prime editing bypasses these problems (see 'Precision editor'). Although it also uses Cas9 to recognize specific DNA sequences — just like CRISPR-Cas9 does — the Cas9 enzyme in the prime editing tool is modified to nick only one DNA strand. Then, a second enzyme called reverse transcriptase and guided by a strand of RNA, makes the edits at the site of the cut.
The prime editing enzymes don't have to break both strands of DNA to make changes, freeing researchers from relying on the cell's DNA repair system — which they can't control — to make the edits that they want. This means that prime editing could enable the development of treatments for genetic diseases caused by mutations that aren't easily addressed by existing gene-editing tools.
A multipurpose tool
Previously, researchers, including Liu, thought that they would need to develop gene-editing tools specific to each category of change they wanted to make in a genome: insertions, deletions or DNA letter substitutions. And the options were limited when it came to making precise substitutions.
An older technique, called base editing, which is comparable in precision to prime editing, chemically converts one DNA letter directly into another — something CRISPR-Cas9 can't do — such as converting a T to an A or a G to a C, without breaking both DNA strands2. Developed by Liu, base-editing could be useful for correcting some genetic diseases caused by single-letter mutations, including the most common form of sickle-cell anaemia.
But base-editing can't help with genetic disorders caused by multi-letter mutations such as Tay-Sachs disease, a usually fatal illness typically caused by the insertion of four DNA letters into the HEXA gene.
So Liu and his colleagues set out to create a precise gene-editing tool that gave researchers the flexibility and control to make multiple types of edits without having to create bespoke systems. In 2018, the team hit on prime editing: a combination of enzymes, including a modified Cas9 enzyme, that could change individual DNA letters, delete letters, or insert a series of letters into a genome, with minimal damage to DNA strands.
"It's fantastic," says Sontheimer. "The breadth of the mutations that can be introduced is one of the biggest advances. That's huge."
But Liu's team and others will now need to carefully evaluate how well the system works in a variety of cells and organisms. "This first study is just the beginning — rather than the end — of a long-standing aspiration in the life sciences to be able to make any DNA change at any position in an organism," says Liu.



Reader Comments
to our Newsletter