However, it's also difficult to keep up with the medical literature, given the sheer number of articles that appear on a daily basis. It's a considerable task to separate the wheat from the chaff of this medical literature. Reading the reports of scientific research itself - compared to reading reviews and case reports - is quite a large task, as it takes a lot of time and effort to form at least an overall assessment of the methodology and statistics used, and to be able to assess whether the research was carried out properly.
And yet that's what I'll try to do here, again. This concerns, of course, data on the Pfizer/BioNTech vaccine against the SARS-CoV-2 virus, which was published online on December 10th on the website of the New England Journal of Medicine.
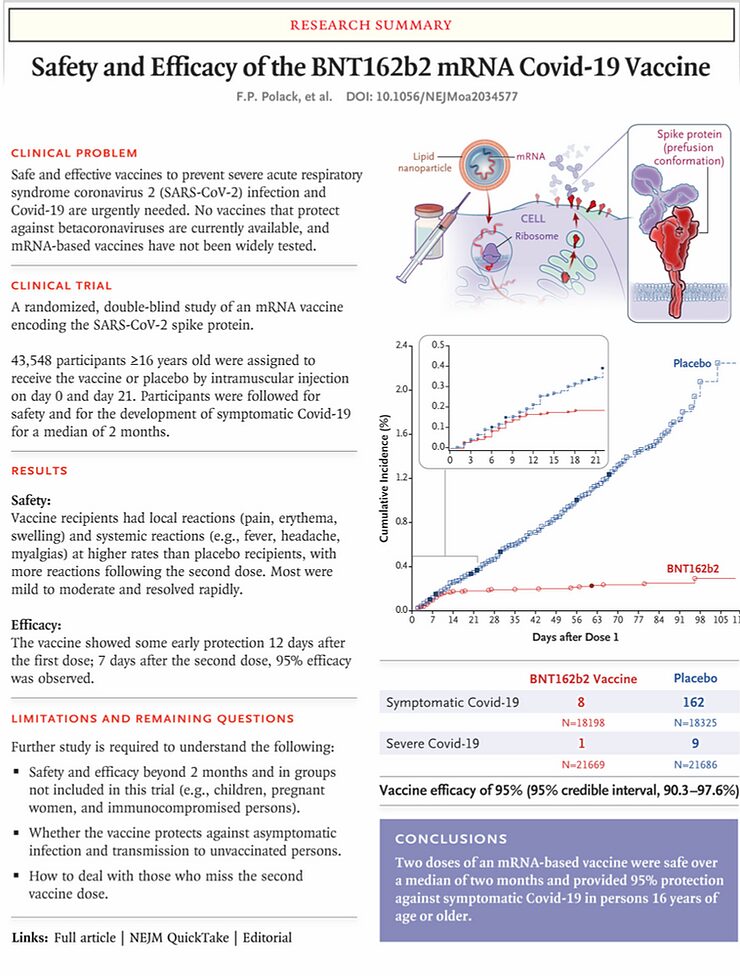
"Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine."
https://www.nejm.org/doi/full/10.1056/NEJMoa2034577?query=featured_home
This Pfizer/BioNTech vaccine against the SARS-CoV-2 virus was approved by the European Medicines Agency on Monday, December 21st, 2020, and will be the first vaccine used in the Netherlands against this virus. Much is expected of it, although until this publication, only the press releases of the manufacturer itself were available, which generally isn't the most reliable source of scientific information.
It's good to calmly list all the facts and data, to see what is known about this vaccine, but especially to also see what isn't yet known. I will start with the abstract of the article because it already raises more questions than answers.
"BNT162b2 is a lipid nanoparticle-formulated, nucleoside-modified RNA vaccine that encodes a prefusion stabilized, membrane-anchored SARS-CoV-2 full length-spike protein"
This is the vaccine we're talking about. It's based on a messenger RNA (mRNA) that is stabilized by changing some of the nucleosides - the building blocks of the RNA - so that the mRNA is not broken down by the body too quickly. This mRNA encodes the spike protein of the SARS-CoV-2 virus, the most immunogenic part of the virus. It's this spike protein that allows the virus to enter a cell by binding to the ACE2 receptor. Subsequently, this mRNA is introduced into (muscle) cells via small particles, which then express the mRNA, and the 'spike protein' of the virus is placed on its own cell membrane, in the same way as it's on the membrane of the virus. There, it's recognized by the cells of the immune system and thus the immune response is triggered. This vaccination technique is new and has never been used before.
Commentaar: See also:
- As Moderna's Covid-19 vaccine takes the lead, its Chief Medical Officer's recent promotion of 'gene-editing vaccines' comes to light
- 'Recipients being misled to a criminal extent': What's not being said about Pfizer's new Coronavirus vaccine
- DNA, mRNA vaccines of the future!
The 'Conclusions' section of the abstract of the paper reads: "A two dose regimen of BNT162b2 conferred 95% protection against Covid-19 in persons 16 years of age or older. Safety over a median of 2 months was similar to that of other viral vaccines." This means that we simply don't know anything about the safety of this vaccine over a period of more than two months. That data simply isn't available.
But that's not all. The introduction of the article states that collecting data from the phase 2/3 studies that deal with the 'immunogenicity' and 'durabilitity' of the immune response - the degree to which the vaccine is able to elicit an immune response and how long this immune response lasts - is still ongoing and not reported in the article. Loosely translated, this means that it's not known whether the vaccine is able to induce a permanent immune response, which would permanently protect the recipient against infection with the SARS-CoV-2 virus.
Who was not allowed to participate in the study?
With any study that looks into the effect of a medicine or vaccine, it's important to know who was, and especially who wasn't, allowed to participate in the study. This is important for the internal validity of the research, but especially for external validity. 'Internal validity' is a term that describes the extent to which the drug or vaccine is effective for people who didn't participate in the study, but correspond in terms of characteristics of the participants in the study. In general, it can be assumed that this is the case.
More important, however, is the concept of 'external validity': The question of whether the findings of the study also apply to people whose characteristics do not correspond with the characteristics of the participants in the study. In other words, can the drug or vaccine be considered to work just as well for people with characteristics other than those of the study participants as it did for the study participants? That's always the question that must be answered before applying the results of scientific research in practice. In short: Do the results of this scientific research apply to the patient sitting in front of me in the consultation room? This is often not the case.
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(04)17670-8/fulltext
It's therefore extremely important in this study to look at the people who were not allowed to participate in the study. The main exclusion criteria for this study were a 'medical history of Covid-19', 'treatment immunosuppressive therapy', or 'diagnosis with an immunocompromising condition'.
Please note, these are probably the people most likely to fear infection with the SARS-CoV-2 virus and want to take the vaccine! This approach has therefore been criticized from various sides because the 'exclusion criteria' were very broadly defined, and the researchers had a large degree of freedom as to who they included and who they didn't include in the study. It may now be assumed that the risk of serious illness and death as a result of an infection with the SARS-CoV-2 virus increases with age, and is also considerably higher in people with multiple underlying conditions, summarized with the term 'comorbidity'.
The researchers who conducted this study:
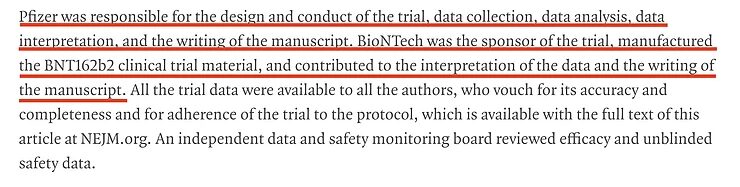
The next thing we need to ask is: who designed, conducted, analyzed and published the study? The answer is quite clear: the manufacturer. This is a study designed, conducted, analyzed, published and paid for by Pfizer/BioNTech itself. The fact that an independent data and safety committee was able to see the data doesn't change this, simply because they had no say in the design of the research, selection of the participants, the statistics used or the publication.
I don't need to explain here how such a construction can lead to biased results of scientific research, because it has been extensively researched and published about over the past twenty years.
https://jamanetwork.com/journals/jama/article-abstract/196846
John Ioannidis, in his monumental publication 'Why Most Published Research Findings Are False' in the PLoS in 2006, pointed out once again in 'Corollary 5':
"The greater the financial and other interests and prejudices in a scientific field, the less likely the research findings are to be true."
https://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.0020124
Comment: For more information on John Ioannidis, see:
- World's leading epidemiologist John Ioannidis on COVID-19 fiasco: 'We are making decisions without reliable data'
- Modelers were 'astronomically wrong' in COVID-19 predictions, says epidemiologist Dr. John Ioannidis - And the world is paying the price
- Stanford U. epidemiologist John Ioannidis lambasts the media for panicking the public over Covid-19
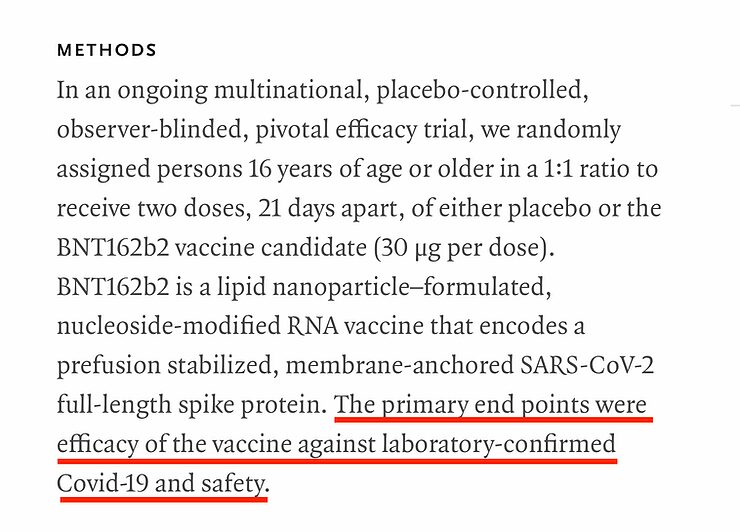
The primary and secondary end points of the study:
Primary and secondary 'end points' in a medical study are a symptom or set of symptoms that are used to determine whether or not the drug being trialled is effective. In the case of a drug designed to prevent heart attacks for example, a 'primary end point' might be chest pain. If any of the trial participants develop chest pain during the trials, they reach the 'end point' and are removed from the trial. This data on the number of people who developed chest pain (not as a result of the trial but despite it) and those who did not would then be used to determine the efficacy and safety of the drug.
The primary 'end point' of the study on the Pfizer vaccine is defined as follows:
'The onset of COVID-19, by confirmation of a positive result on the RT-PCR'
I won't discuss the unreliability of RT-PCR tests in the diagnosis of COVID-19 here, as it has already been extensively discussed and may be considered as common knowledge. I already described this extensively in my previous blog post, the poor performance of this test in clinical practice has now been described in several well-executed scientific studies.
https://www.janbhommel.com/post/de-verduistering
More important is how they defined COVID-19. The definition is as follows:
"Confirmed COVID-19 was defined according to the Food and Drug Administration (FDA) criteria as the presence of at least one of the following symptoms: fever, new or increased cough, new or increased shortness of breath, chills, new or increased muscle pain, new loss of taste or smell, sore throat, diarrhea, or vomiting, combined with a respiratory specimen [i.e. a positive result from the RT-PCR] obtained during the symptomatic period or within 4 days before or after"Please note, one of these symptoms in combination with a positive RT-PCR result for the SARS-CoV-2 virus was sufficient to diagnose COVID-19. One symptom was enough. The study doesn't state how many symptoms people with COVID-19 had or how severe these signs or symptoms were. This study also didn't look for other (viral) agents of these complaints and symptoms, while at least one study suggests that when COVID-19 is suspected there are often other (viral) agents that could explain the complaints and symptoms. In that study of 50 people, 5 people ultimately had a positive result on the RT-PCR for the SARS-CoV-2 virus, but 6 people had a positive result on the RT-PCR for influenza A or B.
https://www.sciencedirect.com/science/article/abs/pii/S1386653220301165
As far as the definition of COVID-19 in the study is concerned, it goes without saying that these are, without exception, symptoms for which the general practitioner would advise taking a paracetamol and getting under a blanket. Without exception, these are symptoms for which people generally don't even consult a doctor. It's therefore very questionable how relevant the diagnosis of COVID-19 is if it's defined in this way.
However, there was also a secondary outcome measure and that's the occurrence of 'severe COVID-19'. Here too it's not necessary to explain that the only outcome measure that matters is: to what extent is a vaccine against the SARS-CoV-2 virus able to keep people out of the hospital, to what extent is a vaccine able to prevent admission to intensive care and to what extent is the vaccine able to prevent people from dying from the infection? Those are the most important outcome measures, that's what it's all about. I will come back to this later.
Who were allowed to participate in the study?
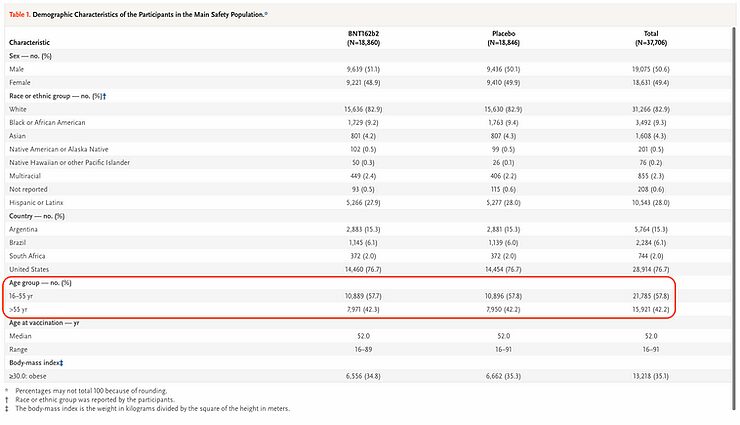
The next step is of course to look at the features and characteristics of the people who were included in the study. Firstly, the age of the participants. Almost 58% of the participants in this study on the SARS-CoV-2 vaccine were between the ages of 16 and 55. It's this age group, almost 2/3 of the participants in this study, who have little to fear from infection with the SARS-CoV-2 virus, and a vaccine is therefore of little value to them. One may even wonder, with what is now known about the Infection Fatality Rate of the SARS-CoV-2 virus, broken down by age, whether it's ethical to include these people in the study. I will come back to that later as well.
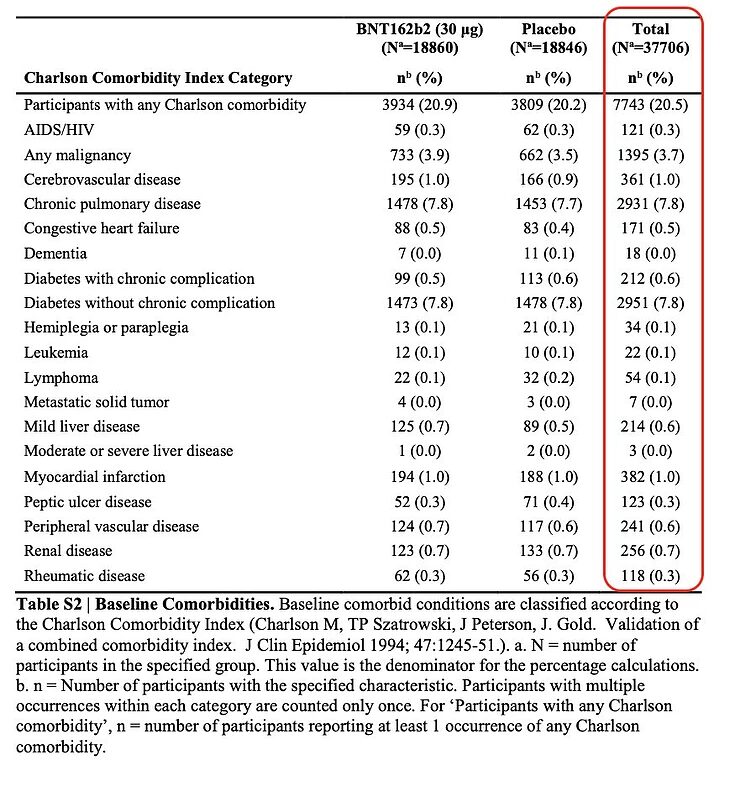
This is also evident from the underlying conditions - comorbidity - of the participants in the study. In total, only one out of five of the people appear to have an underlying condition, and for the various individual underlying conditions, the percentage of people suffering from them is often less than 1%.
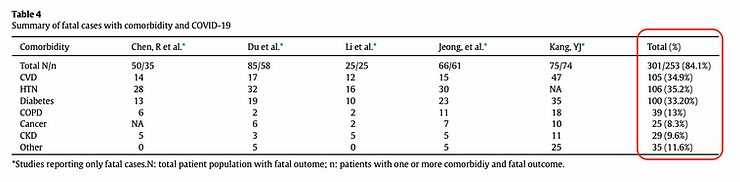
How different is this for people who become seriously ill and die from COVID-19. The table below is from a study on comorbidity in fatal cases of COVID-19. The table shows that 9 out of 10 people have an underlying condition that increases the risk of a severe COVID-19 course.
https://www.ajicjournal.org/article/S0196-6553(20)30637-4/pdf
If people in nursing homes and the elderly are vaccinated, the percentage of people with an underlying condition will be many times higher than among the participants in the study on the Pfizer/BioNTech vaccine. I fear that the comorbidity in this group of people is more along the lines of 80% instead of 20%, and perhaps even higher. The external validity of this study for people in nursing homes and the elderly is therefore strongly limited, because it's precisely these people who didn't participate in the study. And it is precisely these people who fear an infection with SARS-CoV-2 and who have the most to gain from an effective and safe vaccine.
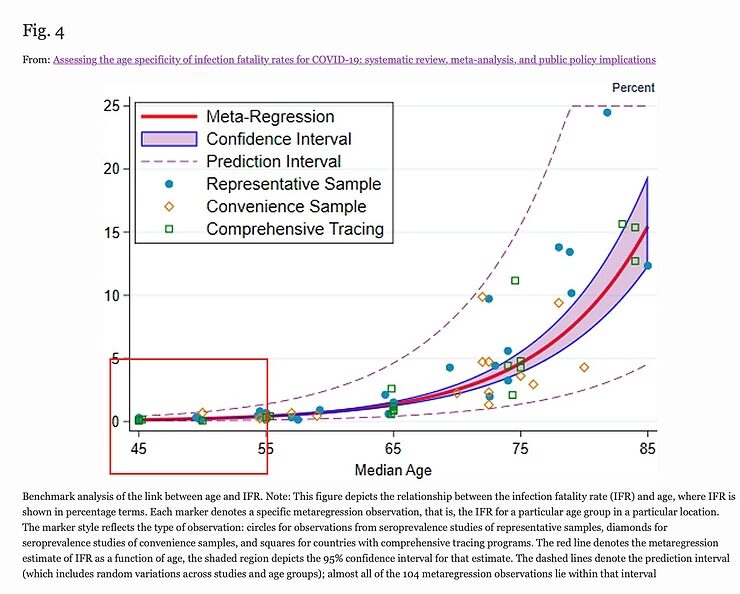
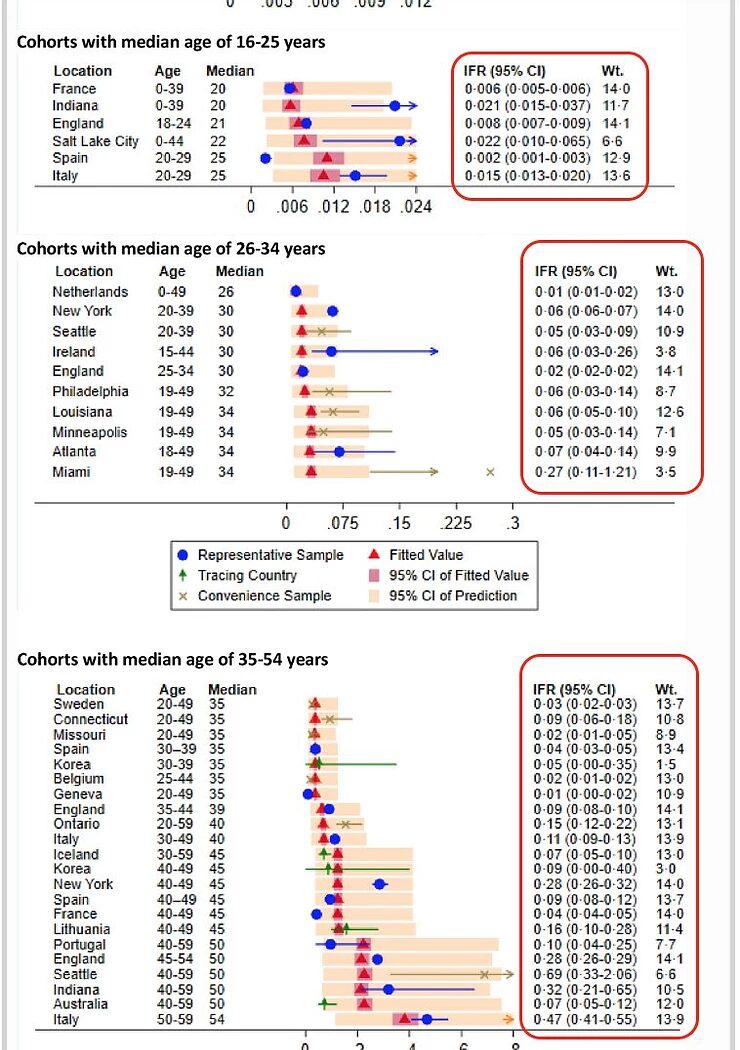
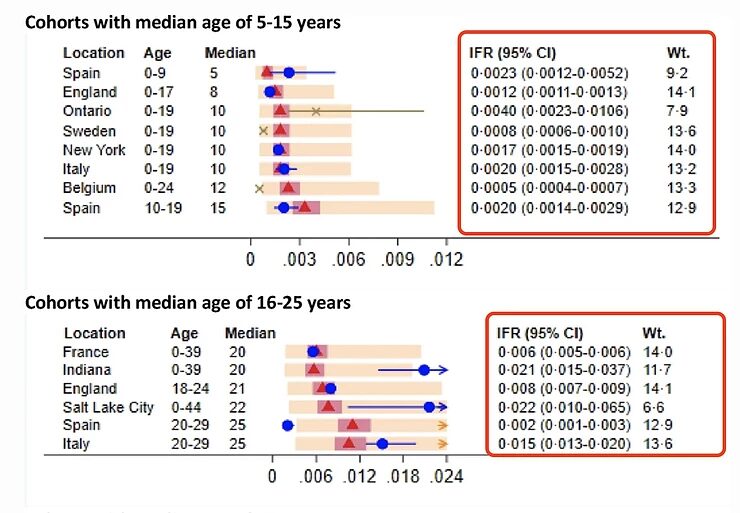
Very recently, an article was published about the age-specific Infection Fatality Rate (IFR) of the SARS-CoV-2 virus. Here, the IFR of an infection of the SARS-CoV-2 virus is broken down into different age groups. Such research has its traps and pitfalls, but it's the best available data we currently have.
https://link.springer.com/article/10.1007/s10654-020-00698-1#Fig4
I'll use two graphs from this study that illustrate my statement that the age group between 16 and 55 has very little to fear from the SARS-CoV-2 virus: the highest estimate of the IFR in the 35-54 age group is 0.4%, but most estimates are between 0.1 and 0.2%. For the younger age groups, the IFR is much lower and for children it is even lower than for Influenza.
Every year in the United States, 100-150 children die from Influenza, a massive number in comparison to the single digit deaths from SARS-CoV-2 virus among children. Even an infection with the Respiratory Syncytial Virus (RS virus) causes many times more fatalities among children than the SARS-CoV-2 virus ever will. https://lci.rivm.nl/richtlijnen/rsv-infectie
This means that in this age group, 1 to 2 people in 1000 people would die from an infection with the SARS-CoV-2 virus, with people with underlying conditions at the greatest risk. A vaccine must already be highly effective and safe if it's to improve the prognosis of these people.
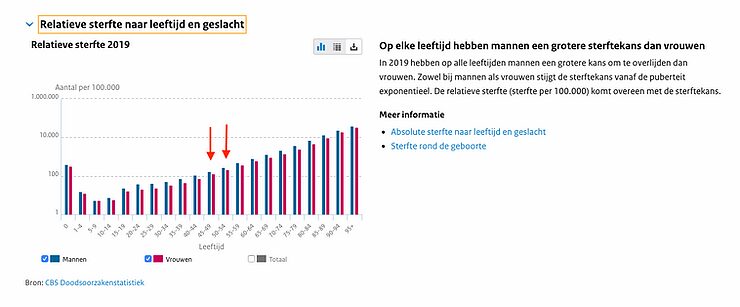
Moreover, if one looks at the age-specific mortality of a 45-year-old, this is also 1 in 1000, and therefore doesn't deviate much from the statistically expected mortality at this age. It cannot simply be said that this doubles the risk of death, because people can only die once.
https://www.volksgezondheidenzorg.info/onderwerp/sterfte/cijfers-context/huidige-situatie#node-relatieve-sterfte-naar-leeftijd-en-geslacht
The side effects of the Pfizer/BioNTech vaccine:
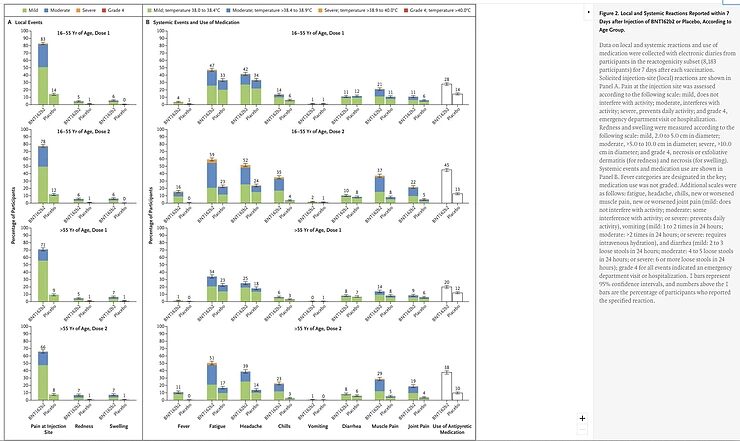
It should come as no surprise that an effective vaccine has more (mild) side effects. The immune system is activated, which can best be compared to a symphony orchestra that's starting to play. Many different cells are activated, T cells and B cells, but also many other cells and all these cells produce signal substances called cytokines that lead to flu-like symptoms that can also occur with an infection with the virus itself.
These side effects can be divided into local and systemic side effects. Especially the local side effect of pain was much more common in the group that was vaccinated, about eight times more compared to the group that received the placebo. The systemic side effects of headache and fatigue were twice as frequent in the vaccinated group.
Although these are relatively mild and transient side effects, these differences in the degree of side effects jeopardize the blinding of the study. It's conceivable, and even plausible, that people who experienced many side effects from the injection assume that they received the vaccine, and were therefore less likely to attribute any nonspecific complaints such as headache, muscle pain and coughing to COVID-19 and therefore didn't report these. This is all the more difficult because it's not described which complaints in either group led to the suspicion of COVID-19 and how frequent and serious these complaints were.
If it turns out that the people who had COVID-19 and received the vaccine reported on average many more, and more serious, complaints than the people in the placebo group who were diagnosed with COVID-19, this would be an indication that the vaccinated group didn't interpret the milder complaints as an expression of COVID-19 and therefore didn't report them. In addition, while the researchers were 'blind' to who received the vaccine and who didn't, they were not 'blind' to the registration of side effects, and this may have influenced the decision whether or not to test someone. I also want to mention that the people who administered the vaccines or placebo were not 'blind'. The question is, therefore, to what extent did they interact with the researchers?
As for the side effects themselves, it's entirely justifiable for people to have such mild complaints from a vaccine if it's to prevent a serious and potentially fatal infection. And one may wonder if this is the case. The mortality among the 21,728 people who received the placebo is exactly... zero!
The results of the study:
These are the results of the effect of the vaccine against the SARS-CoV-2 virus on the primary outcome measure as described above. Indeed, the probability of having mild and non-specific complaints in the vaccinated group is 95% lower. However, in my opinion this is an irrelevant outcome measure, of zero value whatsoever. The extent to which a vaccine is able to prevent relatively mild complaints - as are also seen with a mild flu, cold or gastroenteritis - is in my opinion of no value, while the vaccine is approved and authorized on the basis of these data. Ultimately, it concerns 169 people with these complaints in the placebo group and 9 people in the vaccinated group.
"Confirmed COVID-19 was defined according to the Food and Drug Administration (FDA) criteria as the presence of at least one of the following symptoms: fever, new or increased cough, new or increased shortness of breath, chills, new or increased muscle pain, new loss of taste or smell, sore throat, diarrhea, or vomiting, combined with a respiratory specimen [i.e. a positive result from the RT-PCR] obtained during the symptomatic period or within 4 days before or after"Please note! Let me say it again, just one of these non-specific complaints, as mentioned above, was enough to give the clinical diagnosis of COVID-19.
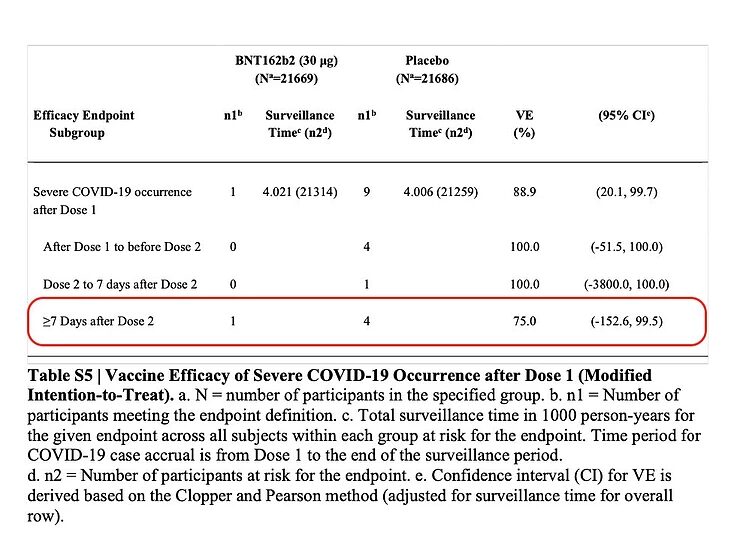
Of course I would like to know to what extent the vaccine is able to prevent 'severe COVID-19'. Unfortunately, that's not mentioned in the article and I have to look for it in the supplement of the article. If you look at 7 days from the 2nd dose of the vaccine, it appears that there were four cases of severe COVID-19 in the placebo group and one in the vaccinated group.
Now there is a number called the 'Number Needed to Vaccinate', and while this is understood to be different concepts in the literature, it's illustrative to show here how many people must be vaccinated to prevent one case of 'severe COVID-19'. That's 19,965/(4-1) = 6555 people. Of course, this Number Needed to Vaccinate will drop as more people get sick, and what's more, these numbers are nothing more than statistical noise.
An important point, however, is that we don't know how many people in the placebo group actually had an infection with the SARS-CoV-2 virus. What could easily have been carried out as a parallel study is mapping how many people in the placebo group developed antibodies in order to estimate how many people actually had an infection with the SARS-CoV-2 virus in the period of the study, to determine which proportion of people were still 'at risk' of getting the infection. However, this has not been done, and in my opinion this is a major shortcoming of this study. It's easy to understand from the perspective of the producer, who wants to sell as many vaccines as possible. It's not in his interest to show that a large proportion of people no longer need their vaccine because they have already been infected with the SARS-CoV-2 virus.
Furthermore, I find it extremely striking that the authors dare to state on the basis of these figures that the 'theoretical' probability of 'vaccine-mediated disease enhancement' is negligible. With such a low frequency of the disease, this is a very premature conclusion, since it's a rare phenomenon.
The safety of the vaccine:
What do the authors write about the safety of the vaccine? They say there's a 83% probability of discovering a relevant side effect if the side effect in question occurs more often than in 1 in 10,000 vaccinees (0.01%). This also automatically means that there is a 17% probability that such an adverse side effect will not be detected at this frequency of occurrence. Rarer side effects, or side effects that only occur at a later stage, cannot of course be found with this research.
Commentaar: See also: Disaster: 20% of Moderna's human test subjects sustained severe injuries from Gates-Fauci coronavirus vaccine
What I find particularly disturbing, and I don't understand why the editors of the NEJM approved this, is that the authors of the article argue that it would be (ethically) unjustified to assess the safety and efficacy of the vaccine in the context of continuing the double blind study. This implies that they intended to also vaccinate the placebo group. Doing this will definitely eradicate the possibility to attribute any serious side effects to the vaccine with certainty. This directly serves the interest of the vaccine producer in the context of any liability, even if this liability has been deposited with the national authorities.
Comment: See also: You can't sue Pfizer or Moderna if you have severe Covid vaccine side effects. The government likely won't compensate you for damages either
In addition, I wonder why one should break the blinding and why it wouldn't be ethical to continue the study in its original setup, since the Infection Fatality Rate (IFR) of the study participants is very low, and the question remains whether the vaccine will benefit them. That's precisely the research question of the study in the long term.
What I wonder above all is why Pfizer/BioNTech apparently thinks it's ethical to vaccinate children under the age of 12 years in a research context against the SARS-CoV-2 virus, an infection that doesn't make, or barely makes, children sick. Plus, the probability that they can die from it is virtually zero. The probability that children will die from an infection with the SARS-CoV-2 virus is in all likelihood lower than the probability of dying from an infection with the Influenza virus.
I am therefore downright baffled that at such an extremely low Infection Fatality Rate in these age groups, Pfizer/BioNTech apparently received permission from the various authorities to vaccinate children in a research context with an experimental vaccine that, as mentioned, has not been used before.
The authors go too far again in the discussion, and I fear this sentence comes directly from Pfizer/BioNTech's PR department, stating the following: "The rigorous demonstration of safety and efficacy less than 11 months later... "
However, nothing 'rigorous' has been demonstrated yet, the results are no more than an indication, the final results will take years to come.
What is the end conclusion?
Here are the pertinent questions that a proper study into the efficacy and safety of a vaccine should answer:
- What is the effect of the Pfizer/BioNTech vaccine on the number of hospital admissions, the number of intensive care admissions and mortality?
- What is the long-term effectiveness of the vaccine, in this case a period longer than two months?
- What is the long-term safety of the vaccine, in this case a period longer than two months?
- What do we know about rarer but possibly serious side effects, such as autoimmune disorders, which can also occur in the longer term and for which this research didn't have the required duration and power?
- Is the vaccine able to break the chain of transmission, that is, prevent the transmission of the virus from one person to another?
What is now being rolled out is a massive vaccine experiment unlike anything seen before, with only minimal data on the safety and efficacy of the vaccine used. It's quite possible that in a few years time it will be concluded that this new mRNA technique has led to a safe and effective vaccine, but such a conclusion now is extremely premature and extremely risky. It would not be the first time that major accidents have occurred as a result of haste and carelessness, as was recently rightly pointed out in an editorial in JAMA. https://jamanetwork.com/journals/jama/fullarticle/2766651
Also, the fact that the Pandemrix vaccine against the swine flu, which was promoted by Ab Osterhaus (among others) as a 'killer virus', was on closer inspection discovered to be one of the most benign influenza viruses ever, should warn us against great haste and alarming carelessness. But apparently politicians, administrators, many academics - and yes, many doctors too - are deaf and blind to the lessons history should have taught them.
Finally, I would like to suggest that the highly educated ladies and gentlemen - legal scholars, ethicists and philosophers, people such as Roland Pierik, Marcel Verweij, Gert van Dijk, Brigit Toebes and Martin Buijsen - first thoroughly immerse themselves in the matter before they try to forcibly push the vaccine into the arms of the Dutch people, like true vaccine-fascists. The uncritical propaganda for a vaccine that has not yet remotely proven its value, effectiveness and safety is extremely detrimental to the use of other vaccines that have proven their safety and efficacy and actually plays into the hands of the very people who categorically reject any form of vaccination.
Translated by Sott.net
Jan B. Hommel is a Dutch neurologist. His blog can be found here.










Regards the safety - I did not see any studies out there on epitope homology with the spike protein (multiple sequences, alternate sequences, contiguous sequences, conformational binding (3D) homology, etc) to human tissue. This could cause immuno-pathologies due to amino-acid sequence binding similarities. There was a concern that the spike trimer has certain homologies with syncytin-1 and 2 (also a trimeric protein) that is responsible in mediating placenta binding to the uterus, and for suppressing the female immune system attacking the foetus. Without these proteins the foetus would miscarry quickly. I have not done a full study on this.
Interestingly, syncytin-1 and 2 are produced from genes acquired from ancient integration of viral DNA into the mammalian genome.