There follows an open letter from 66 doctors, scientists and clinical practitioners to the Royal College of Obstetricians and Gynaecologists (RCOG), the Royal College of Midwives (RCM) and the U.K. Health Security Agency (UKHSA) regarding safety concerns about COVID-19 vaccinations in pregnancy. Where is the evidence, they ask.Obstetricians and gynaecologists in the U.K. have put their faith in and adjusted their practice according to guidance from their Royal College (RCOG). However, recent advice from the RCOG has been in complete contradiction to everything that it itself and academic institutions have been teaching about evidence-based medicine.
This advice is that: COVID-19 vaccines are not only safe but strongly recommended for pregnant women.Such advice is not grounded in robust data based on ethically conducted research - and anyone who is medically and academically trained should take serious issue with this.
Ethics of clinical research
Clinical researchers, especially when conducting trials to investigate pharmaceutical products, are required to update themselves every two years on the principles of Good Clinical Practice, which incorporate the Nuremberg Code and the Declaration of Helsinki. According to those principles, it is unethical to violate a study protocol by under-reporting adverse events, by removing subjects with adverse events from the study and by unblinding study participants prematurely with the purpose of administering the product under investigation to everyone and therefore effectively ending the trial - as have all happened in the COVID-19 vaccine trials. It is unethical to prevent the public from accessing raw trial data for 75 years and to only release some of it for independent scrutiny after a lawsuit. It is unethical to extrapolate the conclusions of a prematurely ended trial to vulnerable groups not represented in the trial - such as pregnant women.
For obvious reasons, pregnant women are usually excluded from clinical trials. The British National Formulary frequently advises against the use of a pharmaceutical product in pregnancy as a precaution due to lack of data. In pregnancy, lack of data is sufficient to be hesitant. Two examples in the not-too- distant past remind us how disastrously wrong it can go when a new product is given to pregnant women: thalidomide caused severe limb defects in the foetus, and diethylstilbestrol (DES) increased the risk of certain cancers after exposure in utero, requiring life-long surveillance for more than one generation. It was indeed the thalidomide scandal which led to the establishment of the U.K. Yellow Card system for adverse event reporting. But suddenly all of this seems to be forgotten.
Lack of robust and reliable safety data
A recent public controversy focused on MHRA advice updated on August 16th 2022 stating in the toxicity conclusions that
"sufficient reassurance of safe use of the vaccine (mRNA BNT162b2/Pfizer/BioNTech) cannot be provided at the present time" and "women who are breastfeeding should also not be vaccinated."The Government and the RCOG were very quick to express their concerns about the circulation of this apparent misinformation and to reinforce their advice that pregnant women should get vaccinated. This document was originally from December 2020, and so the claim is that this section is outdated. The question remains why this section was not amended if this document was recently updated. The answer is of course because there is nothing to update it with: studies regarding genotoxicity, carcinogenicity, reproductive and developmental toxicity, and prenatal and postnatal development have still not been conducted.
It cannot possibly be known whether it is safe to give these products to pregnant and breastfeeding women. Clinical research standards dictate close and prolonged observation of trial subjects, documenting any and all observed clinical effects following administration of the trial compound. This has not been done. There are no trials that last even the duration of a pregnancy. COVID-19 vaccines were on the market for a mere four months when the initial advice to avoid them in pregnancy changed by 180 degrees and they were declared safe. Potential adverse effects for the offspring have not even been considered.
It is profoundly unethical to give a completely novel compound to pregnant women on a mass scale without the strict protocols of clinical research to just see what happens and then pretend that this is science. Yet this is exactly what has been happening.
Incorrect interpretation of available data
Safety data are largely based on retrospective and observational cohort analyses and registries, such as the CDC's V-Safe COVID-19 Vaccine Pregnancy Registry. Voluntary registries are not equivalent to well-designed prospective clinical trials, as follow-up is inconsistent and incomplete with no standardisation or systematisation and no tracking of participants.
Other data are from short-term studies where outcomes are determined in post hoc analyses, with little or no stratification of gestational age at the time of vaccination. A large Canadian study published in the Lancet concluded that "COVID-19 vaccines have a good safety profile in pregnancy" based on a follow-up period of a whole seven days. Conflicts of interest status on this paper is notable. Publications are clearly biased towards reaching the conclusions of affirming safety and effectiveness of COVID-19 vaccines in pregnancy even when their study data do not allow such conclusions. The U.K. Medical Freedom Alliance (UKMFA) has published on its website open letters to the U.K.-based authors of two such studies with a critique of their conclusions. Both papers were widely propagated to the public.
The systematic review and meta-analysis of the effectiveness and perinatal outcomes of COVID-19 vaccination in pregnancy was co-authored by the current president of the RCOG, who shared this headline with the RCOG membership: "COVID-19 vaccination associated with 15% reduction in stillbirths in pregnant women." The prompt within the message to "Find out more" linked not to the original paper for everyone to scrutinise and recognise the flawed methodology, but to the Guardian propagating the same headline. The work of Professor Norman Fenton (Professor of Risk Information Management) on the "statistical illusion of better pregnancy outcomes for vaccinated women" is worth considering for a comprehensive analysis of the available data.
Currently, any quantitative assessment of the risks of adverse events in pregnancy is mostly stymied by the lack of reliable denominators, prohibiting accurate interpretation of existing data.
Shimabukuro et al. published their preliminary findings of mRNA COVID-19 vaccine safety in pregnancy in the NEJM based on the V-Safe registry, reporting a miscarriage rate of 12.6% - consistent with the general population. This was based on a denominator of 827 completed pregnancies. The conclusion was incorrect as only 127 women had been vaccinated in the first or second trimester, and so by definition the remaining 700 women could not possibly have had an early pregnancy loss.
According to post-marketing data from Pfizer, 42,086 adverse events were reported to the manufacturer during the first three months of the vaccination programme. Amongst these were reports from 270 pregnant women. Only 32 pregnancy outcomes were recorded. This should have been but indeed was not a study with dedicated follow-up. These data were collected as part of post-marketing surveillance and are insufficient for comprehensive analysis.
Therefore, there are no reliable statistics at this time - but there are plausible mechanisms of potential harm and there are glaring safety signals.
Mechanisms of potential harm
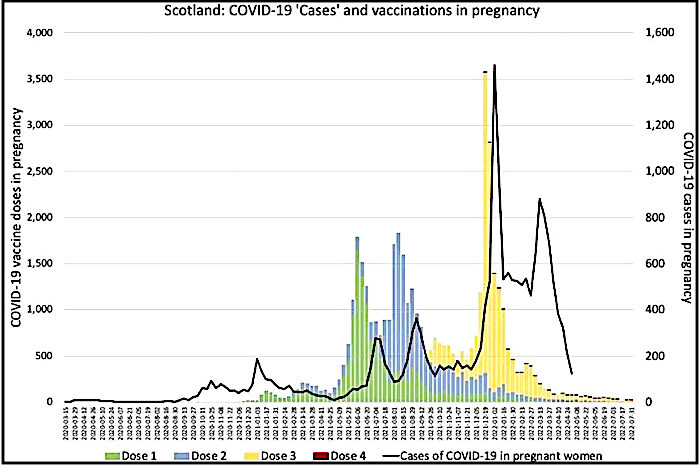
Even if pregnant women were at increased risk from COVID-19, there are no conclusive data demonstrating that those risks are mitigated by vaccination. Regarding effectiveness, it is worth considering the data tracking COVID-19 vaccination and infection in pregnancy in Scotland, which do not indicate vaccination to have been beneficial, indeed they suggest quite the opposite (Figure 1).
Independent of the potential risks to the pregnancy itself, there are now well-acknowledged risks of COVID-19 vaccines for women of childbearing age in general, including risks of cardiac and cardiovascular morbidities, which may well affect a pregnancy.
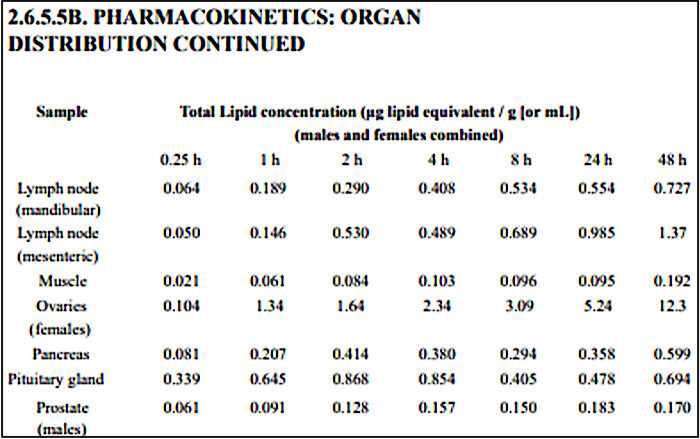
Pfizer's own pharmacokinetics studies showed that the lipid nanoparticles used to carry the mRNA are distributed to and accumulate in the ovaries at significant concentrations (Table 1).
A recent research letter in JAMA Pediatrics highlighted that COVID-19 vaccine mRNA could be detected in breast milk. The clinical significance of this has not been investigated, but the conclusion advises caution against breastfeeding for the first 48 hours after vaccination, and previous studies have described adverse events in 7.1% of breastfed infants.
A study published in PLOS Pathogens showed that in mice "the mRNA-LNP vaccine platform induces long-term immunological changes, some of which can be inherited by the offspring". The effect on the immune system in human offspring - including defence against infections as well as the propensity to allergies and autoimmune disorders - is at this stage completely unknown.
Concern regarding potential autoimmunity is also based on molecular mimicry. mRNA vaccines induce human cells to produce antigens (spike proteins) in order to elicit an immune response. Similarities between spike protein and human proteins may lead to an adverse autoimmune reaction. It is potentially relevant for pregnant women that the SARS-CoV-2 spike glycoprotein was found to share similarities with 27 human proteins that relate to oogenesis, uterine receptivity, decidualisation and placentation in a study published in the American Journal of Reproductive Immunology.
Safety Signals
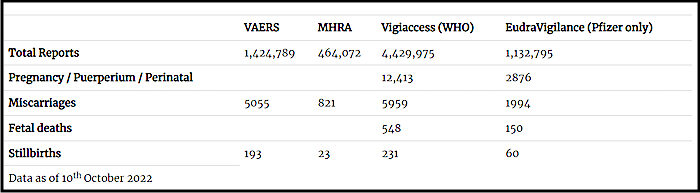
Most concerning are the accumulating safety signals - and the apparent reluctance to fully investigate them. All four major databases for adverse event reporting (VAERS, MHRA Yellow Cards, EudraVigilance, WHO Vigiaccess) contain significant numbers of pregnancy-related adverse outcomes, including miscarriages and stillbirths (Table 2).
A study - currently in preprint - by Dr. James Thorp (U.S. specialist in foeto-maternal medicine) compares pregnancy-related adverse outcomes reported after COVID-19 vaccination to those reported after influenza vaccinations. Even considering the limitations of the study and the perhaps questionable validity of this comparison, the number of reports following COVID-19 vaccines of miscarriages, foetal chromosomal abnormalities, foetal malformation, foetal cystic hygroma, foetal cardiac disorders, foetal arrhythmia, foetal cardiac arrest, foetal vascular mal-perfusion, foetal growth abnormalities, foetal abnormal surveillance, foetal placental thrombosis, low amniotic fluid and foetal death and stillbirth are extremely concerning.
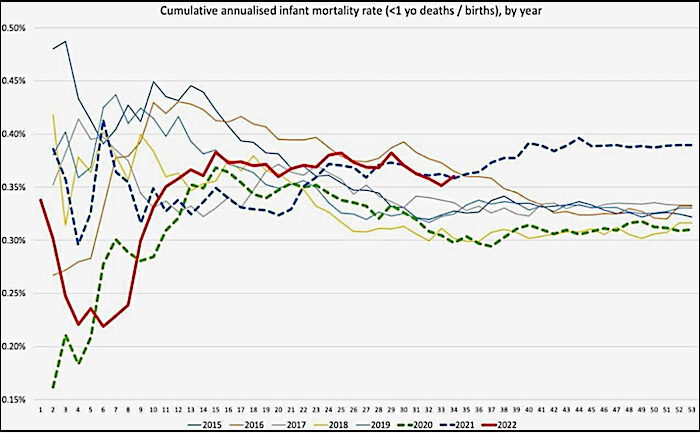
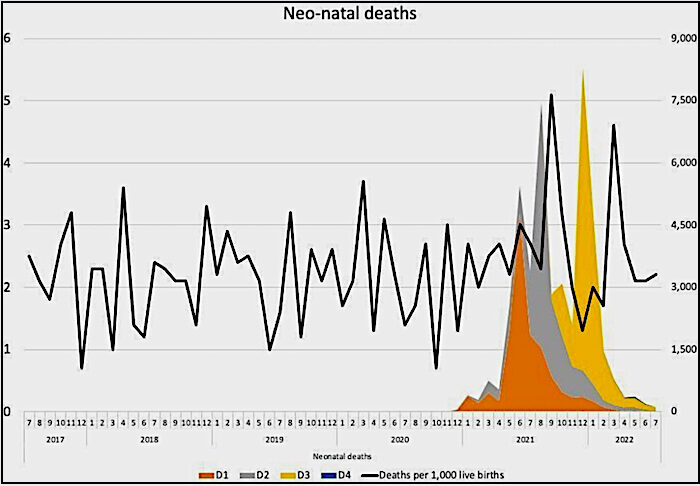
In addition, there are reports of unexplained phenomena. Birth rates in the first half of 2022 appear to have fallen significantly in highly vaccinated countries in Europe based on official figures, with a decline of more than 4% in 15 countries and more than 10% in seven countries. The rates of cumulative annualised infant mortality in Scotland show 2021 as a significant outlier (Figure 2). As the data are cumulative, the variation usually evens out towards the end of the year, but not so in 2021. The rise mostly relates to spikes in neonatal deaths, which have occurred in temporal association with COVID-19 vaccination (Figure 3). This correlation is especially remarkable considering not all pregnant women were vaccinated.
These spikes in neonatal deaths have been publicly acknowledged as concerning. Dr. Sarah Stock, expert in maternal and foetal medicine at the University of Edinburgh, commented in May 2022: "The numbers are really troubling, and I don't think we know the reasons why yet", but "stressed the Covid vaccine, which studies have consistently shown to be safe in pregnancy, was not a factor". This cannot possibly be known unless it is investigated without the bias that has afflicted most publications on this subject to date. The need for investigation is urgent, and whilst this should have been with clinical trials, there should now be a moratorium on COVID-19 vaccines to allow for meticulous retrospective analysis and re-evaluation.
If we continue to ignore these safety signals, we are not doing our due diligence to protect patients from harm. According to the principles of Good Medical Practice outlined by the General Medical Council, we are supposed to take action when we are concerned about compromised patient safety.We look forward to an early response to our concerns.
We are not just concerned but deeply disturbed and alarmed at the widespread distortion of science and the blatant omissions in the process of bringing a newly developed pharmaceutical product to market.
We have a collective duty to restore the principles of medical ethics to our practice and to clinical research to protect the most vulnerable groups from harm, and this includes pregnant women and their babies.
In the absence of data on long-term outcomes of mRNA COVID-19 vaccination in pregnancy for either women or their infants, vaccination of pregnant women should be paused while a full safety enquiry is conducted and until results of long-term studies on animals as well as pregnant women and their offspring firmly and unequivocally establish that the benefits of vaccination clearly outweigh the risks to both mothers and babies.
- Dr Julia Wilkens, FRCOG, MD, Consultant in Obstetrics & Gynaecology
- Dr John Williams, FRCOG, retired Consultant in Obstetrics & Gynaecology
- Professor Angus Dalgleish, MD, FRCP, FRACP, FRCPath, FMed Sci, Principal, Institute for Cancer Vaccines & Immunotherapy (ICVI)
- Professor Richard Ennos, MA, PhD, Honorary Professorial Fellow, University of Edinburgh
- Professor John Fairclough, FRCS, FFSEM, retired Honorary Consultant Surgeon
- Professor Dennis McGonagle, PhD, FRCPI, Consultant Rheumatologist, University of Leeds
- Professor Karol Sikora, MA, MBBChir, PhD, FRCR, FRCP, FFPM, Honorary Professor of Professional Practice, Buckingham University
- Lord Moonie, MBChB, MRCPsych, MFCM, MSc, retired member of the House of Lords, former parliamentary under-secretary of state 2001-2003, former Consultant in Public Health Medicine
- Dr Victoria Anderson, MBChB, MRCGP, MRCPCH, DRCOG, General Practitioner
- Julie Annakin, RN, Immunisation Specialist Nurse
- Helen Auburn, Dip ION MBANT NTCC CNHC RNT, registered Nutritional Therapist
- Dr David Bell, MBBS, PhD, FRCP(UK), Public Health Physician
- Dr Mark A Bell, MBChB, MRCP(UK), FRCEM, Consultant in Emergency Medicine
- Dr Michael D Bell, MBChB, MRCGP, retired General Practitioner
- Dr Alan Black, MBBS, MSc, DipPharmMed, retired Pharmaceutical Physician
- Dr Gillian Breese, BSc, MB ChB, DFFP, DTM&H, General Practitioner
- Dr H Burger, MRCGP, DRCOG, General Practitioner
- Dr David Cartland, MBChB, BMedSci, General Practitioner
- Caroline Cartledge, RM, BA (hons), Midwife
- Angela Chamberlain, BSc (hons), Midwife
- Dr Peter Chan, BM, MRCS, MRCGP, NLP, General Practitioner, Functional Medicine Practitioner
- Michael Cockayne, MSc, PGDip, SCPHNOH, BA, RN, Occupational Health Practitioner
- James Cook, NHS Registered Nurse, Bachelor of Nursing (Hons), Master of Public Health (MPH)
- Dr Clare Craig, BMBCh, FRCPath, Pathologist
- Dr David Critchley, BSc, PhD in Pharmacology, 32 years' experience in Pharmaceutical R&D
- Dr Sue de Lacy, MBBS, MRCGP, AFMCP, UK Integrative Medicine Doctor
- Dr Jayne LM Donegan, MBBS, DRCOG, DCH, DFFP, MRCGP, General Practitioner
- Dr Jonathan Eastwood, BSc, MBChB, MRCGP, General Practitioner
- Dr Elizabeth Evans, MA(Cantab), MBBS, DRCOG, Co-founder UKMFA
- Dr Christopher Exley, PhD FRSB, retired Professor in Bioinorganic Chemistry
- Dr John Flack, BPharm, PhD, retired Director of Safety Evaluation, Beecham Pharmaceuticals, Senior Vice-president for Drug Discovery SmithKline Beecham
- Sophie Gidet, RM, Midwife
- Dr Ali Haggett, Mental health community work, 3rd sector, former Lecturer in the history of medicine
- Dr Keith Johnson, BA, D.Phil (Oxon), IP Consultant for Diagnostic Testing
- Dr Rosamond Jones, MBBS, DRCOG, MD, FRCPCH, retired Consultant Paediatrician
- Dr Tanya Klymenko, PhD, FHEA, FIBMS, Senior Lecturer in Biomedical Sciences
- Dr Caroline Lapworth, General Practitioner
- Dr Branko Latinkic, BSc, PhD, Reader in Biosciences
- Dr Theresa Lawrie, MBBCh, PhD, Director, Evidence-Based Medicine Consultancy Ltd, Bath
- Dr Felicity Lillingstone, IMD, DHS, PhD, ANP, Doctor, Urgent Care, Research Fellow
- Dr Geoffrey Maidment, MBBS, DRCOG, MD, FRCP, retired Consultant Physician
- Dr Ayiesha Malik, MBChB, General Practitioner
- Dr Kulvinder S. Manik, MBChB, MRCGP, MA(Cantab), LLM, Gray's Inn
- Dr Franziska Meuschel, MD, ND, PhD, LFHom, BSEM, Nutritional, Environmental and Integrated Medicine
- Dr Graham Milne, MBChB, DRCOG, MRCGP, General Practitioner
- Dr David Morris, MBChB, MRCP(UK), General Practitioner
- Margaret Moss, MA(Cantab), CBiol, MRSB, Director, The Nutrition and Allergy Clinic, Cheshire
- Theresa Ann Mounsey, BSc (hons) in midwifery studies
- Dr Sarah Myhill, MBBS, Naturopathic Physician, retired General Practitioner
- Dr Chris Newton, PhD, Biochemist working in immuno-metabolism
- Dr Rachel Nicholl, PhD, Medical Researcher
- Sue Parker Hall, certified transactional analyst (CTA, psychotherapy), MSc (Counselling & Supervision), MBACP (senior accredited practitioner), EMDR practitioner, Psychotherapist
- Rev Dr William J U Philip, MBChB, MRCP, BD, Senior Minister The Tron Church, Glasgow, formerly doctor working in cardiology
- Anna Phillips, RSCN, BSc Hons, Clinical Lead Trainer Clinical Systems (Paediatric Intensive Care)
- Dr Angharad Powell, MBChB, General Practitioner
- Dr Jessica Righart, MSc, MIBMS, Senior Biomedical Scientist
- Mr James Royle, MBChB, FRCS, MMedEd, Colorectal Surgeon
- Dr Salmaan Saleem, General Practitioner
- Dr Rohaan Seth, BSc (hons), MBChB (hons), MRCGP, retired General Practitioner
- Dr Noel Thomas, MA, MBChB, DObsRCOG, DTM&H, MFHom, retired Doctor
- Dr Livia Tossici-Bolt, PhD, Clinical Scientist
- Tanya Wardle, RM, Registered Midwife
- Dr Helen Westwood, MBChB, MRCGP, DCH, DRCOG, General Practitioner
- Dr Carmen Wheatley, DPhil, Orthomolecular Oncology
- Mr Lasantha Wijesinghe, FRCS, Consultant Vascular Surgeon
- Dr Lucie Wilk, MD, Consultant Rheumatologist




HELLOOO.... ANYONE ON THIS PLANET CAN HELP US ?