A Japanese Health Ministry panel has said a coronavirus vaccine developed by Daiichi Sankyo can be used for inoculations in Japan. It would be the first Japanese-made Covid shot available for practical use.Would you be surprised to know that the majority of the panel still wears masks?
The panel of experts held a meeting on Monday. The experts said they confirmed the vaccine's effectiveness, and that they have no serious concerns over its safety.Banzai! Unfortunately, the article doesn't give any details of the effectiveness the experts apparently confirmed. Fortunately, Daiichi-Sankyo previously put out a press release joyously announcing that the primary endpoint had been reached in the Phase Three trial of their Covid vaccine (DS-5670). So...did DS-5670 reach 95% effectiveness like Pfizer/BioNTech and Moderna did?
The primary endpoints of the booster vaccination trial were the geometric mean blood neutralising antibody titer and immune response against SARS-CoV-2 (Omicron BA.5 subvariant) four weeks after the investigational vaccination. Results on the endpoints in the DS-5670 group were higher than those in the control group (Omicron-adapted bivalent vaccine approved in Japan), statistically demonstrating non-inferiority of DS-5670.In other words, DS-5670 produced more antibodies against BA.5 than the Pfizer/BioNTech or Moderna bivalent jab, neither of which has ever been shown to be effective. How many more? Dunno yet.
Detailed results from the booster vaccination trial will be presented at academic conferences and in publications.You may be thinking, "Hang on. Are you telling me that Japan just approved a completely new Covid jab based on unpublished antibody titers?" Yep, that's exactly what I'm telling you.
But Daiichi-Sankyo is using the same mRNA platform as Pfizer/BioNTech and Moderna, so at least the Ministry of Health, Labour, and Welfare (MHLW) is not green-lighting new technology for them...
Rise of the Replicons
Unlike for Meiji Seika Pharma, where the MHLW is green-lighting new technology for a second new Covid jab. From the company's press release:
Meiji Seika Pharma Co., Ltd. announced today that it has received approval for the manufacturing and marketing of 'Kostaive™ for Intramuscular Injection' (ARCT-154), a self-amplifying mRNA vaccine against COVID-19, from the Ministry of Health, Labour and Welfare (MHLW) in Japan.This is the world's first approval of a self-amplifying mRNA vaccine. So what's a self-amplifying mRNA vaccine? An mRNA vaccine that self-amplifies, obviously.
The mRNA in Kostaive is designed to self-amplify once delivered into cells, so that it generates a strong immune response and the potential for extended duration of protection while using lower doses of mRNA compared to existing mRNA vaccines.In other words, your cells can become even more efficient mRNA factories. The below image and description come from an article in the top immunology journal Cell ominously titled 'Rise of the RNA machines - self-amplification in mRNA vaccine design'. Note that self-amplifying mRNA can also be called replicon RNA.
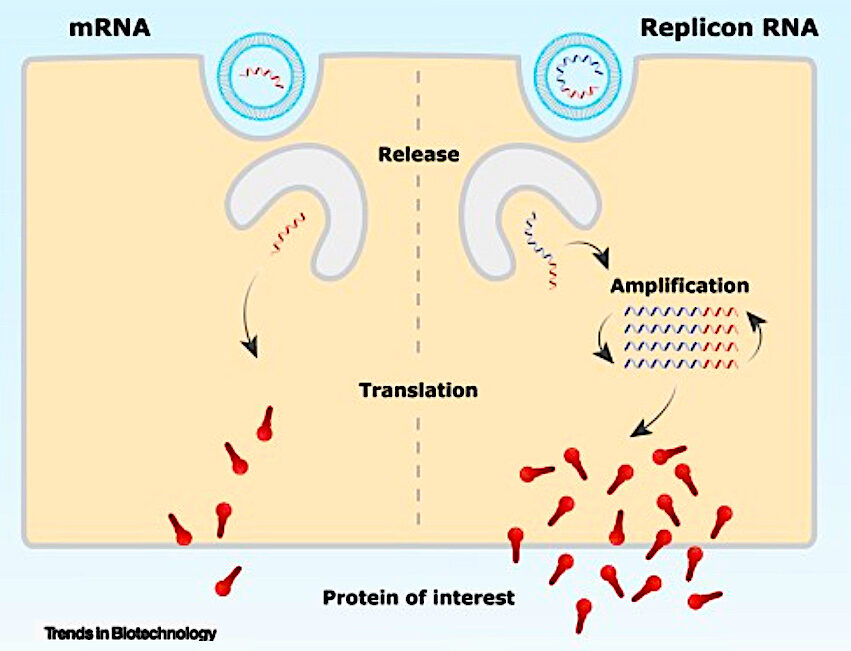
Once released in the cell, the mRNA is translated to produce the protein of interest. In contrast to mRNA, replicon RNA encodes alongside the protein of interest, self-amplifying genes (depicted in blue) that amplify the replicon RNA. This intracellular amplification will subsequently result in higher expression levels of the protein of interest.I don't claim expertise here, but I doubt this is going to fix the problem of spike protein overproduction seen with the original mRNA platform. The Cell article even suggests as much.
At present, the main challenges involved in the global authorisation are potential safety concerns regarding the replicative character of these vaccines... For example, replicon vaccines could persist in immunocompromised individuals as clearance may be less efficient.Because no other self-amplifying mRNA vaccine has previously been approved, the MHLW at least demanded the company do a large-scale randomised controlled trial to test the jab's real-world effectiveness against infection and severe disease, right? Haha, no. Back to the press release.
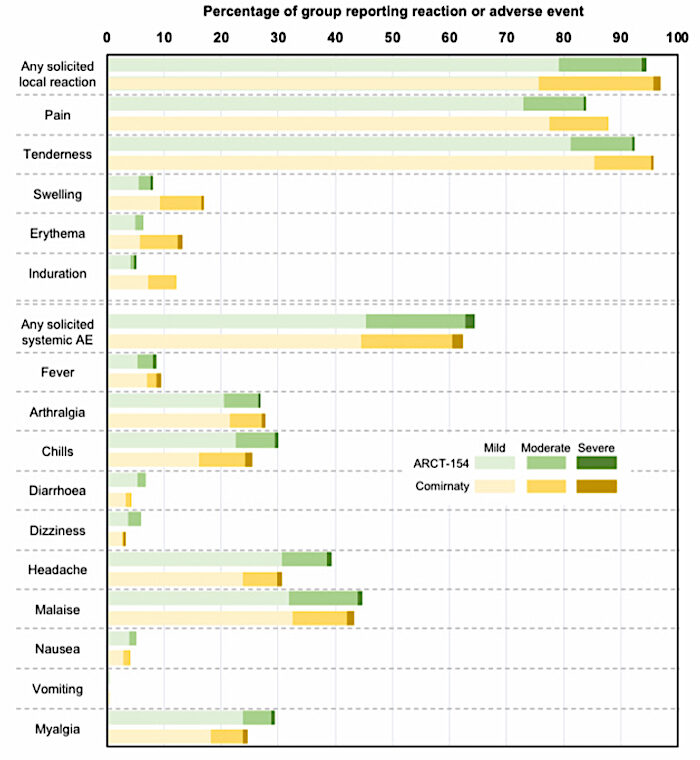
In the Phase III clinical trials for booster vaccination conducted in Japan, 5 μg Kostaive™ elicited higher (p<0.05) and longer-lasting neutralising antibody titers against the original strain, as well as the Omicron BA 4-5 subvariant, compared to 30 μg COMIRNATY ® , a licensed conventional mRNA vaccine targeting COVID-19.At least this time there's a pre-print available (here). Whereas the Phase Three trial for Pfizer/BNT's Comirnaty started with about 40,000 unvaxed participants, this one had only 828, who'd all had three shots previously.
Such a small-scale trial clearly can't tell us if the new platform causes any of the same safety issues associated with the original platform let alone any different ones (which is kind of the point). But Kostaive (ARCT-154) interestingly still managed to, er, amplify the rates of chills, headaches and myalgia (muscle aches and pains) compared with Comirnaty despite one sixth of the dosage. That's the magic of self-amplification, I guess.
However, the version of Kostaive in the trial targeted the wild-type virus that went out of circulation in early 2021, so Meiji Seika Pharma won't release that one. Instead, the company plans to release an updated version of Kostaive in 2024 and is currently conducting another Phase Three trial of a bivalent version, again comparing antibody titers.
So not only did the MHLW approve a vaccine that uses new technology based on antibody titers, it didn't even approve it based on antibody titers from the vaccine that will actually be released. The old definition of 'regulation', like those of 'safe' and 'effective', appears to have died due to Covid.
This seems to be one of the enduring legacies of the COVID-19 pandemic: regulatory agencies have dropped the pretence of doing their jobs properly and will happily rubber stamp any application for approval from favoured companies on the basis of essentially meaningless data. Despite what they claim, the MHLW's experts haven't confirmed that the new jabs offer any benefit to anyone other than the vaccine makers, but they have confirmed that they themselves certainly don't.




Comment: The threat within: Doing the job nature won't.