Scientists have created an entirely new type of ice that neither floats nor sinks — and more closely resembles a liquid than frozen water.
It could even hold clues to life beyond Earth by offering an insight into the processes that shape the oceans of Saturn and Jupiter's moons, where some scientists believe extra-terrestrial organisms may exist.
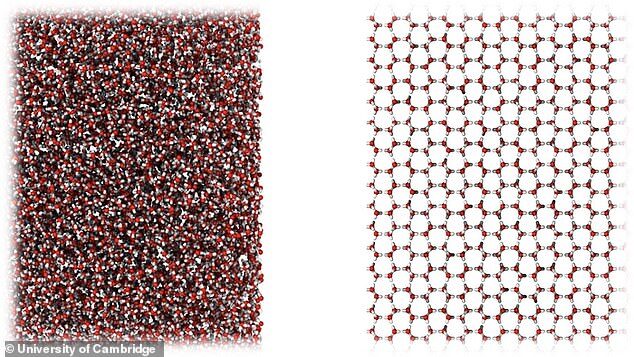
The new form of ice is amorphous, which means that unlike ordinary crystalline ice - in which the molecules arrange themselves in a regular pattern - its molecules are in a disorganised form and resemble a liquid.
The theory is that if this ice does exist there, perhaps in cracks in ice sheets, it could have implications for potential alien life.
This is because one of the properties of the new type of ice is that it stores a lot of energy in its creation - and releases a large amount in its destruction. This burst of energy has a knock-on effect on how tectonics might work on these moons, as well as potentially for extra-terrestrial organisms.

: 'Water is the foundation of all life.That's because the density of liquid water lies in the middle and therefore scientists thought it impossible for ice to form at that density.
'Our existence depends on it, we launch space missions searching for it, yet from a scientific point of view it is poorly understood.
'We know of 20 crystalline forms of ice, but only two main types of amorphous ice have previously been discovered, known as high-density and low-density amorphous ices.
'There is a huge density gap between them and the accepted wisdom has been that no ice exists within that density gap.'
But researchers found that the ice produced in their experiment had a density right between the other two known forms of amorphous ice - at almost exactly the same density as liquid water.
They named this new goldilocks type of ice medium-density amorphous ice (MDA).
In their experiments, the UCL and Cambridge researchers used a process called ball-milling — vigorously shaking ordinary ice together with steel balls in a jar cooled to -200 degrees Centigrade. Rather than ending up with small bits of ordinary ice, the process yielded a novel amorphous form of ice.
Co-author Professor Andrea Sella, of UCL, said: 'We have shown it is possible to create what looks like a stop-motion kind of water. This is an unexpected and quite amazing finding.'
Professor Salzmann added:
'Our study shows the density of MDA is precisely within this density gap.Professor Angelos Michaelides, lead author from Cambridge's Yusuf Hamied Department of Chemistry, said: 'Amorphous ice in general is said to be the most abundant form of water in the universe.
'This finding may have far-reaching consequences for our understanding of liquid water and its many anomalies.'
'The race is now on to understand how much of it is MDA and how geophysically active MDA is.'
The research has been published in the journal Science.
What are the 19 other forms of ice?
- Ice Ih - Normal hexagonal crystalline ice. Virtually all ice in the biosphere is ice Ih, with the exception only of a small amount of ice Ic.
- Ice Ic - A metastable cubic crystalline variant of ice. The oxygen atoms are arranged in a diamond structure. It is produced at temperatures between -143°C and -53°C, and can exist up to -33°C, when it transforms into ice Ih. It may occasionally be present in the upper atmosphere.
- Ice II - A rhombohedral crystalline form with highly ordered structure. Formed from ice Ih by compressing it at temperature of -83°C to -163°C. When heated, it undergoes transformation to ice III.
- Ice III - A tetragonal crystalline ice, formed by cooling water down to -23°C at a pressure of 300MPa. Least dense of the high-pressure phases. Denser than water.
- Ice IV - A metastable rhombohedral phase. It can be formed by heating high-density amorphous ice slowly at a pressure of 810MPa. It doesn't form easily without a nucleating agent.
- Ice V - A monoclinic crystalline phase. Formed by cooling water to -20°C at 500MPa. Most complicated structure of all the phases.
- Ice VI - A tetragonal crystalline phase. Formed by cooling water to -3°C at 1.1GPa. Exhibits Debye relaxation.
- Ice VII - A cubic phase. The hydrogen atoms' positions are disordered. Exhibits Debye relaxation. The hydrogen bonds form two interpenetrating lattices.
- Ice VIII - A more ordered version of ice VII, where the hydrogen atoms assume fixed positions. It is formed from ice VII by cooling it below 5°C.
- Ice IX - A tetragonal phase. Formed gradually from ice III by cooling it from -65°C to -108°C, stable below -133°C and pressures between 200MPa and 400MPa. It has density of 1.16 g/cm3, slightly higher than ordinary ice.
- Ice X - Proton-ordered symmetric ice. Forms at about 70GPa.
- Ice XI - An orthorhombic, low-temperature equilibrium form of hexagonal ice. It is ferroelectric. Ice XI is considered the most stable configuration of ice Ih. The natural transformation process is very slow and ice XI has been found in Antarctic ice 100 to 10,000 years old. That study indicated that the temperature below which ice XI forms is −33°C.
- Ice XII - A tetragonal, metastable, dense crystalline phase. It is observed in the phase space of ice V and ice VI. It can be prepared by heating high-density amorphous ice from -196°C to about -90°C at 810MPa. It has a density of 1.3g/cm3 at -146°C (i.e., approximately 1.3 times more dense than water).
- Ice XIII - A monoclinic crystalline phase. Formed by cooling water to below -143°C at 500MPa. The proton-ordered form of ice V.
- Ice XIV - An orthorhombic crystalline phase. Formed below -155°C at 1.2GPa. The proton-ordered form of ice XII.
- Ice XV - The proton-ordered form of ice VI formed by cooling water to around -193°C to -165°C at 1.1GPa.
- Ice XVI - The least dense crystalline form of water, topologically equivalent to the empty structure of sII clathrate hydrates.
- Ice XVIII - A form of water also known as superionic water or superionic ice in which oxygen ions develop a crystalline structure while hydrogen ions move freely.
- Ice XIX - Another proton-ordered form of ice VI formed by cooling water to around -173°C at approximately 2GPa.




I fully expect 7-11 to acquire the rights.