After months of litigation, a
U.S. court has ordered that, by the end of this month, the U.S. Centers for Disease Control and Prevention ("CDC") must produce the first batch of over 19 months' worth of data collected from tens of millions of v-safe participants during the Covid-19 vaccination program.
The V-safe program includes over
137 million entries that have been made following Covid vaccination.
Informed Consent Action Network ("ICAN") was founded by
Del Bigtree to investigate the safety of medical procedures, pharmaceutical drugs, and vaccines while educating the public about their right to informed consent:
"Our goal is to put the power of scientifically researched health information in your hands and to be bold and transparent in doing so, thereby enabling your medical decisions to come from tangible understanding, not medical coercion."
In December 2021, ICAN announced that it was suing to obtain data from v-safe, the new smartphone-based program that the CDC deployed to track adverse events following Covid-19 vaccination.V-safe was launched on 13 December 2020,
the day before Covid injections were made available to the American public. The
CDC website advises that reporting side effects and adverse events can either be done on
v-safe or the
Vaccine Adverse Event Reporting System ("VAERS").
According to the CDC, v-safe allows users to "quickly and easily share with CDC how you, or your dependent, feel after getting a Covid-19 vaccine."
The data from v-safe is "collected, managed, and housed on a secure server by Oracle," a private technology company, in the form of "aggregate de-identified data" - meaning it does not contain personal health information.
This means the data could be shared with the public, just as it is shared with Oracle.
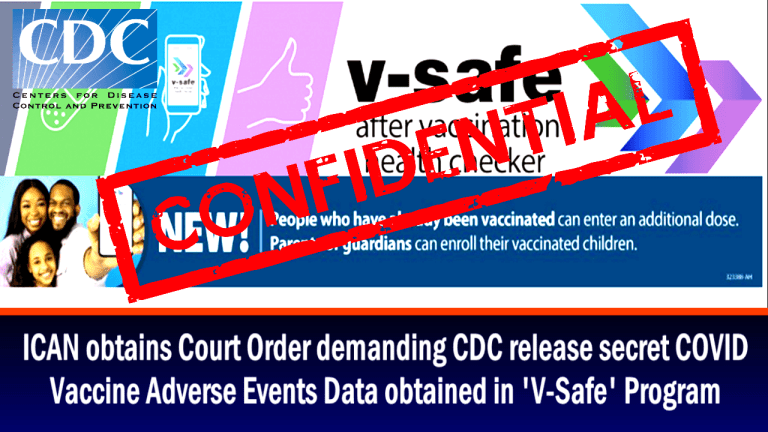
CDC: V-safe After Vaccination Health Checker
In June 2021, ICAN, through its attorneys, headed by Aaron Siri,
submitted legal demands for the v-safe data - specifically demanding, "All de-identified data submitted to v-safe since January 1, 2020."
Despite the CDC's ability to immediately release this de-identified, the CDC took the position that "the information in the app is not de-identified" and so would not produce it. Thus, in December 2021, ICAN sued.ICAN also submitted an
additional request to address the agency's objection, demanding, "All data submitted to v-safe since January 1, 2020," and when the CDC still refused to release the data,
ICAN sued again.
Now, after months of litigation, the court has ordered that, by 30 September 2022, the CDC must produce the first batch of over 19 months' worth of data collected from tens of millions of v-safe participants between 14 December 2020 and 31 July 2022.This is a huge win for ICAN and for the American public, who will finally start to be able to see for themselves the actual self-reported nationwide data about the safety of the Covid-19 injections.

Reader Comments
[Link]
the first batch of 19 months’ worth of data collected from millions of participants who reported adverse events related to COVID-19 vaccination via the V-safe app between Dec. 14, 2020, and July 31, 2022.
just imagine the total HATE HEADED TOWARDS PEDO PETE, CDC, WHO, ESPECIALLY NAZI SHIT STAIN MAGGOT FAUCI,
In all, the CDC will be required to release more than 137 million health V-safe entries.
THATS 137 MILLION MINIMUM TOTALLY FUKD UP FOR LIFE COS OF BEING FORCED THE DEPOP SHOT,
PARENTS THAT FORCED THERE KIDS, YOUR ALL GOING TO HELL,
However, ICAN said, the CDC “had apparently not read its own documentation regarding V-safe” and refused ICAN’s requests, claiming “information in the app is not deidentified.”
THE CDC BOSS, CEO, AND ALL EMPLOYEES, ARE GUILTY AS SIN IN THIS, AGAIN, HELL AWAITS THEM ALL,
PLUS THE OTHER SHIT STAINS, THAT COMPETED, THERE IS NO FORGIVING, GOD REMEMBERS ALL,
YOUR ALL WELL AN TRULY GONNA BE REINCARNATED AS A ROACH, THEN MORTEINED OVER AN OVER, PROBABLY
137 MILLION TIMES, TO FEEL EACH INDIVIDUALS PAIN AN SUFFERING, ALL FOR A BUCK, AND TO SPREAD A MASS LIE,
THERE NEVER WAS ANY VIRUS, NEVER ANY DEADLY PLAGUE, YOU LOT RUINED FRIENDSHIPS, FAMILY RELATIONSHIPS, BUSSINESSES, THE ECONOMY, POLLUTED THE OCEANS 92% MORE WITH THE DUMPING OF MASKS AND SYRINGES INTO THE OCEAN ETC..
“With CDC’s reluctance to release this information, one can only imagine that it will not reflect well on the whole COVID-19 vaccination program, especially given irregularities seen with VAERS [the Vaccine Adverse Event Reporting System] reporting and the shifting narrative of the CDC regarding COVID-19 guidance.”
MASS GENOCIDE, MASS MURDER ON A POLITICAL LEVEL, MR GATES, SOROS, AND ALL YOUR FAMILYS WILL BECOME TARGETS FOR THIS THAT YOUVE DONE, AND SHIT STAIN FAUCI, WELENSKY AND YOUR ACOMPLICES, YOUR ALL NOW OFFICIALLY A TARGET OF THE PEOPLE,
PRESIDENTS, PRIME MINISTERS, HEADS OF STATE, HEALTH MINISTERS, THESE SHIT FOR BRAINS SCIENTISTS, ONLY GOOD AT GUESS WORK UNDER A GOV EMPLOYMENT BADGE, YOU ALSO, ARE FUKD, AND TARGETS,
Historically, VAERS has been shown to report only 1% of actual vaccine adverse events.
[Link]
Original Antigenic Sin — The Hidden Danger of COVID Shots
repeated COVID boosters may result in lowered immunity through a process known as “original antigenic sin” or “immune imprinting”
The COVID jab and the flu vaccine are the No. 1 and No. 2 most dangerous injections respectively, based on adverse event reports and payouts from the U.S. Vaccine Injury Compensation Program. Both are also capable of shedding, and both can make you more prone to infection as their protection wears off
White House COVID coordinator Ashish Jha, September 6, stated, “I really believe this is why God gave us two arms, one for the flu shot and the other one for the COVID shot” — a statement that will live on in infamy as one of the most ridiculous comments from a public health official ever uttered
Both Shots Are Associated With Serious Side Effects
“This fall, they’re telling people to line up for the two riskiest and deadliest injections out there. Media are stating that getting the flu shot and the COVID jab at the same time is ‘safe.’ Yet there are absolutely NO data to support such a claim.”
GOD WILLING, I WOULD RATHER DIE FROM THE FLU THAN THESE MAN MADE BULLSHIT DEPOP CLOT SHOTS,
NATURAL ENDING, RATHER A MAN MADE POLITICAL BULLSHIT DECEIVING ENDING,
Vaccine Journal Warns of Serious Side Effects
COVID Jabs Linked to Excess Deaths
The COVID shots also appear to be responsible for the rapid increase in excess deaths around the world. As reported September 8, 2022, by The Defender, the COVID jabs are causing injuries on a scale we’ve never seen before in medical history.
Yet governments around the world are turning a blind eye, BECAUSE MOST OF THESE UNELECTED SHIT STAINED GOT A SAALINE SHOT, I KNEW A NURSE OF THE STATE PREM MED TEAM, SHE SEEN THE SALINE BOTTLE RELABELED, " PFIZER COV-19" ETC.. TO DUPE THE PUBLIC TO VOLUNTEER FOR THE CLOT SHOT, SHE WAS NEAR SICK, SHE QUIT THE END OF THE WEEK,
In 2021, however, the observed number of deaths were “two empirical standard deviations above the expected number.” What’s more, the increase in mortality only started to accumulate after April that year. A similar pattern was also observed for stillbirths, which rose by 11% in the second quarter of 2021.
The figure below illustrates the differences in excess mortality between 2020, the year of the virus, and 2021, the year of the COVID jabs.19 20 Looking at the age groups, we see something very odd. In 2021, excess mortality was highest among 15- to 79-year-olds, yet COVID infection primarily killed the elderly, 70 to 79 years of age, in 2020.
Mortality in age groups 15 to 29, and 50 to 59, during the pandemic, pre-jab, was actually below average, and excess mortality among children was well below average. Yet in 2021, excess mortality went up for all age groups, not just the elderly. This strongly suggests the COVID virus was not a primary contributor, but rather the experimental injections.
In my view, there’s simply no doubt the COVID jabs are causing more harm than good, and combining a reformulated and never tested bivalent COVID booster with a quadrivalent flu shot could potentially be disastrous.
[Link]