But her own 'debunking' has one obvious problem. Her criticism focuses on the claim that the 'placebo' batches actually have many adverse events associated with them, just not in Denmark, where the study was focused. However, the denominators she uses in order to compare the rates of adverse event reports per batch that turn up in VAERS have been chosen arbitrarily.
She says so herself. Thus she writes:
It is important to note that I do not have the 'doses' (dose number) for the relevant yellow vax lots as per the VAERS data. It is a pity. If I had these data, I could provide a much better analysis. For the purposes of this analysis, I assume that the batch sizes per lot are equal [to those in the Danish study]. This might be a very bad assumption, but what can I do? I'm still better than the CDC staff, combined.But if we do not have the number of doses, what is the point of the comparison? Nobody has ever claimed that there were no adverse event reports associated with these batches. In the Danish study, there were in fact four 'yellow' batches that had literally zero adverse events associated with them. But the other 14 merely had relatively few. The issue is the reporting rate.
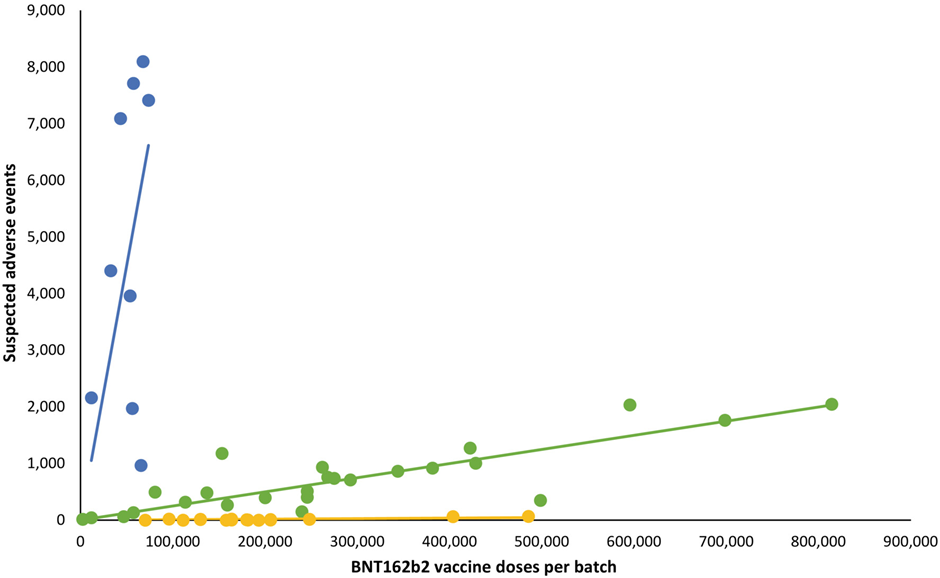
Moreover, the problem is even more serious. As seen in the above quote, Jessica Rose simply assumes that the appropriate denominators for the VAERS reports are equal to the denominators in the Danish study, i.e., the number of doses of each batch deployed in Denmark. But we do in fact know the total number of doses included in the batches in question, and, unsurprisingly, it is far higher. Why unsurprisingly? Well, because the whole is greater than the part.
As discussed in my previous article, these are EU batch releases falling under the authority of Germany's Paul Ehrlich Institute (PEI) as the responsible regulator. Needless to say, the number of doses deployed in just one - moreover, small - EU country is usually going to be less than the total.
In his interview with Milena Preradovic (which is the source of the entire controversy), the German chemistry professor Gerald Dyker notes that, per information provided by the PEI, the total number of doses contained in a batch or lot was "a little less than 1.4 million". Assuming batch releases have been consistently of this volume, this means that the actual number of doses in the EU batch releases is anywhere from nearly three times (FH8469) to nearly 20 times (FM3289) the totals assumed in Jessica Rose's analysis.
Redoing the analysis with the appropriate denominators would presumably make most or all of the 'extra' adverse events reporting that Jessica Rose discovered in VAERS disappear. The total number of reports would remain the same, of course, but they would now be associated with a far higher number of doses. I will leave it to others to undertake the exercise if they see fit.
Jessica Rose appears not to have been aware of the whole-and-part issue in conducting her analysis, i.e., that Denmark is in fact part of the VAERS sample. She thus notes in a footnote that none of the relevant reports in VAERS originated from Denmark. But what she is saying, more precisely, is that none of the reports are listed as having originated from Denmark. This is because almost none of the relevant reports contain any information on filing location at all.
Not surprisingly, since these are EU batch releases, they are almost all foreign (i.e., non-U.S.) reports, and the location simply appears as such - 'foreign' - in top-level VAERS searches. But what is interesting to note is that when Jessica Rose did a search for filing location using a more advanced ('split type') search parameter, she came up with just a few far-flung locations like Brazil, Montenegro, Japan, Moldova, etc. - but not a single EU country! These are EU batches. So, where are the EU countries? In fact, they are undoubtedly all the rest of the reports.
Why then would they not be listed as such? Well, it will be recalled that last November, on the request of 'European regulators', VAERS was purged of EU data. The purged data includes precisely the country codes. See the below notice, which comes directly from the CDC website.
This is yet another of many reasons why VAERS is an extremely blunt instrument to use to try to validate or invalidate the results of the Danish researchers. VAERS is not a 'global' reporting system. It is an American reporting system, which, under specific circumstances, also includes foreign reports which the manufacturers are required to forward to it.
The way to try to validate or invalidate the Danish results is not by digging around in the highly fragmentary VAERS foreign report data, but rather the old-fashioned way dictated by the scientific method. Other researchers in other, preferably EU, countries with access to comparable and similarly complete data, including both adverse event reports and number of doses deployed, have to try to reproduce the results.
It is worth stressing that it is precisely due to the intervention of unnamed 'European regulators' that the VAERS data are even more fragmentary and unhelpful than they would otherwise have been. And it is none other than the release of the batches by one very important European regulator, Germany's Paul Ehrlich Institute, that is at issue in this whole affair.
For, as Gerald Dyker revealed in his interview with Milena Preradovic, not only did the PEI approve all the very bad blue batches for release, it did not even bother to perform quality-control testing on all-but-one of the seemingly very 'good' yellow ones. It was this fact - not the Danish study per se - that led Dyker to suggest that there might indeed be something to the placebo hypothesis.
Finally, Jessica Rose also claims to have provided support for the theory that the Danish findings are merely a result of undetected age-confounding. Thus, she compares the 'worst' blue batch in the sample to one randomly chose yellow batch and finds that the mean age of the blue-batch recipients is significantly higher than the mean age of the yellow-batch recipients.
It should be noted in passing that by choosing the 'worst' blue lot, she has obviously biased the outcome of the comparison. After all, the very premise of the exercise is that we should expect to see more adverse event reports the higher the age.
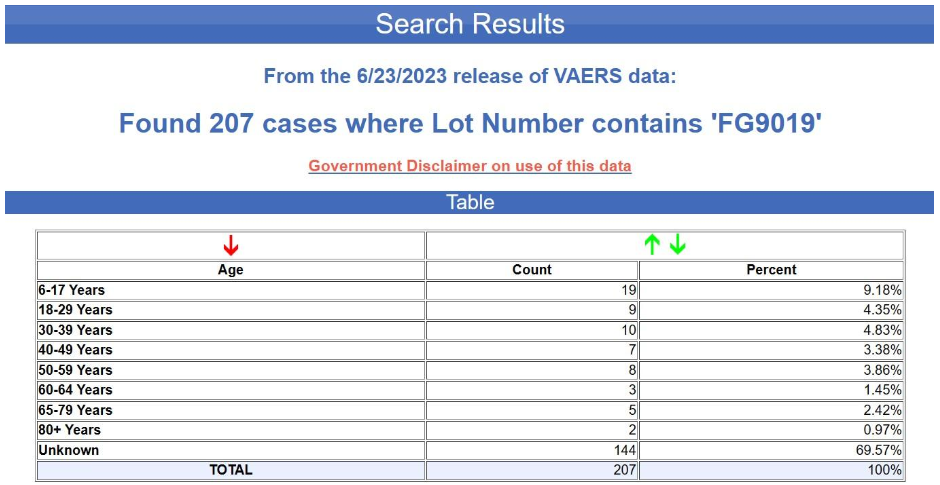
But even supposing that this bias were corrected by including more lots or using more comparable lots, the more fundamental problem here is again the extremely fragmentary character of the data. In fact, the age of the recipient is not even indicated in the overwhelming majority of the reports in question. The tabular search results for the yellow batch (FG9019) are shown below. The age is unknown in nearly 70% of the cases.
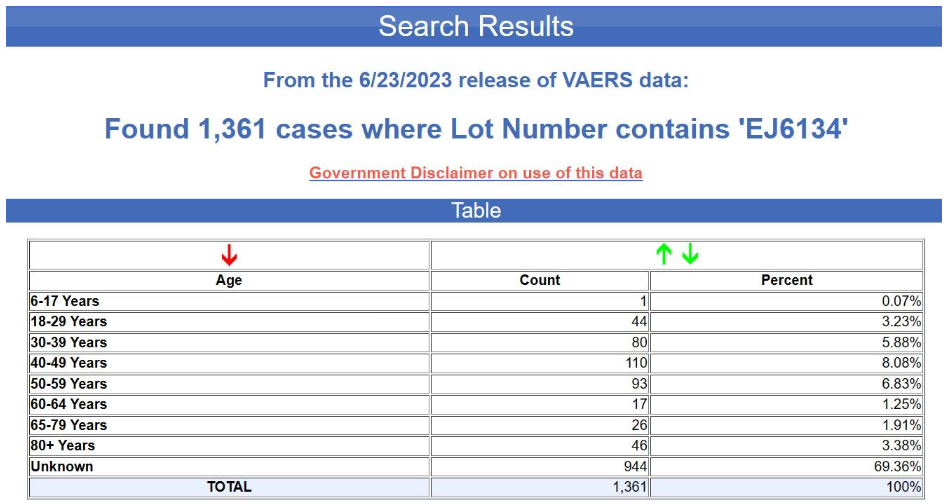
And here are the results for the blue batch (EJ6134).
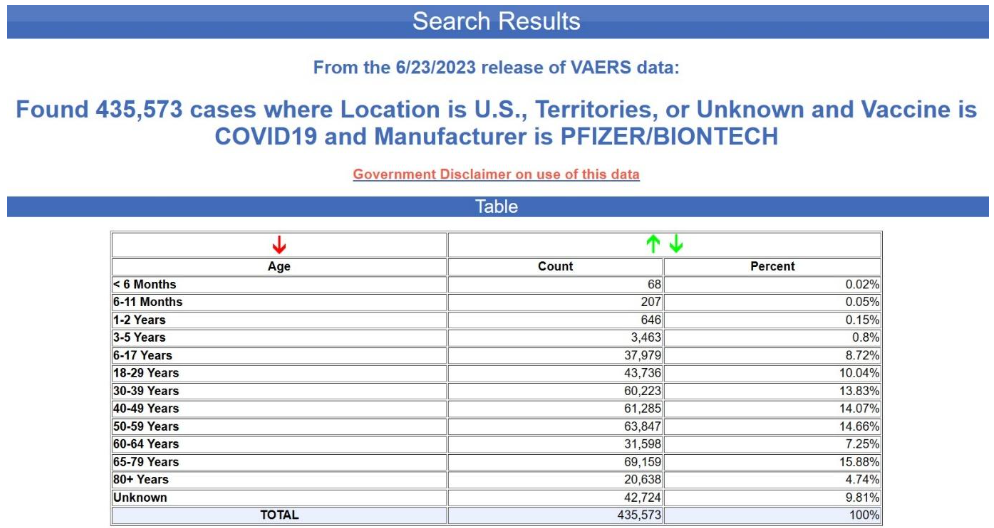
Yet again, the age is unknown in nearly 70% of the cases. This is not at all typical for VAERS, by the way. Below is the age distribution for all the adverse events reports for the Pfizer-BioNTech COVID-19 vaccine excluding the foreign reports i.e., for U.S. reports only.
The age is unknown is only 10% of the reports. Although not acknowledged in the CDC notice, it would appear that the recipient's age has also been scrubbed from most or all of the EU reports. How can one draw conclusions about age-confounding using a database from which most of the age data have been scrubbed?
In any case, if one suspects that the batch variability in the Danish study is just a function of the ages of the vaccinees, why not ask the authors if they have relevant information? As it so happens, they do, and Vibeke Manniche, one of the authors, has addressed the issue in the below tweet reply to Denis Rancourt. The reality is exactly the opposite of what is suggested by the age-confounding hypothesis: more elderly people got the 'safe' yellow batches than the dangerous blue ones.
Robert Kogon is a pen name for a widely-published financial journalist, translator and researcher working in Europe. Subscribe to his Substack and follow him on Twitter.





Reader Comments
It paints a pretty clear picture of differently dangerous lots existing, even back then, so I can understand her presumptions.