© unknown
Kris Homme, a retired engineer, did not know what was happening to her. At age 33, she was diagnosed with macular degeneration - a disease that
usually does not appear until old age. Not one to give up, she somehow managed to complete two graduate degrees with impaired vision. Then, in her 40s, she developed chronic fatigue and multiple chemical sensitivities.
"I was pretty much housebound for a couple years," she recalls. "I just didn't have the strength to leave the house by myself. I was able to keep my house fragrance-free but I had trouble being in a crowd, like on a bus or in an audience where you're sitting next to people because so many people wear fragrances. Or walking on the streets, the car exhaust would be overpowering."
A friend suggested her problem might be mercury exposure from her dental fillings, but she dismissed the idea. After all, her neurologist had already tested her blood for mercury and did not find anything to worry about.
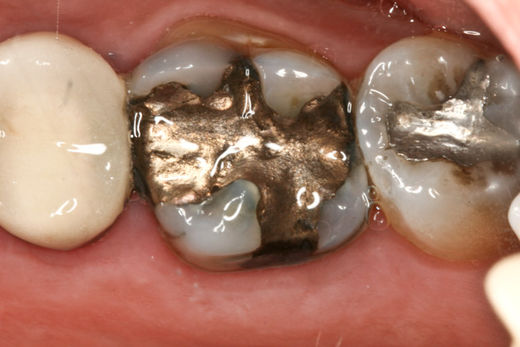
Homme had a mouth full of amalgam fillings, each of which is 50 percent mercury.
The mercury in them was long thought to be inert, but scientists later discovered that some of the mercury is released as vapor and absorbed into the body. Still, the Food and Drug Administration and the American Dental Association maintained they were perfectly safe.
As an engineer, Homme cannot be easily fooled. Even when telling her own story, she repeatedly questions why anyone would want to hear one story when it cannot constitute proof of anything. Knowledge and facts come from carefully controlled, randomized, statistically significant scientific studies, not anecdotes and stories.
The theory that amalgam fillings caused mercury poisoning "all sounded so flaky," she remembers. "The anti-mercury movement has a lot of unfortunate bedfellows so I dismissed the argument."
What's more, she had a degree in Environmental Health Sciences from UC Berkeley. "I just thought that if that was an issue it would have been covered in my prestigious degree program."
Finally, her friend gave her a book to read,
Amalgam Illness, Diagnosis, and Treatment by Andrew Hall Cutler. "I stayed up late, reading and crying. All my symptoms fit and all the theory fit, the theory about how it's not going to show up in a blood test because you're retaining it, you're not excreting it. My whole world turned upside down when I realized my doctors and dentists were so wrong and my degree program was so inadequate and it was like, if I can't believe any of that, what is true? Who can I believe?"
Today Homme is one of several plaintiffs in a lawsuit against the FDA, demanding it respond to several petitions that ask it to ban - or at least seriously restrict - the use of amalgam fillings. Other plaintiffs include the International Academy of Oral Medicine and Toxicology, Moms Against Mercury, and the Cooperative Food Empowerment Directive (CoFED), as well as several individuals. She's also published a peer-reviewed paper summarizing new studies demonstrating the harm of amalgam fillings.
The FDA's strongest evidence of the safety of amalgam fillings are two studies published in 2006 called the "Children's Amalgam Trials." One was conducted in
New England, the other in
Portugal. In them, hundreds of healthy children with low levels of mercury and lead, plenty of unfilled cavities and no previous amalgam fillings were divided into two groups. One group received amalgam fillings, and the other received composite fillings. The children were then monitored over a period of years for changes in mercury levels, IQ, memory and several other neurological tests. They also tracked major health problems in the children over the course of the study.
Both studies found higher levels of mercury in the urine of children who received amalgam fillings, but, on average, they found no significant differences in neurological development and function between the two groups. The New England study also tested kidney function and found no significant differences between the two groups.
But reanalysis of the data from these studies show that perhaps the amalgam fillings were not so benign.
As Homme points out,
humans differ both in their exposure to mercury and their susceptibility to it. When a person who is highly susceptible to mercury is exposed to enough of it, he or she gets sick - even if the same dose would not cause problems for someone who is less susceptible.Scientists have already identified several genes that cause increased susceptibility to mercury. One of them is called CPOX4. A
2012 study looked at a subset of 330 children from the Children's Amalgam Trial conducted in Portugal and found that about 28 percent of them had the susceptible variant of the CPOX4 gene.
Rather than simply averaging the results of the amalgam group and the composite group, the researchers looked at the correlations between urinary mercury levels and neurological test results. Among boys (but not girls) who had the CPOX4 gene variant, the researchers found several significant neurobehavioral deficits associated with increased mercury exposure.
Three other studies also re-examined the data from the Portugal study.
One found evidence that amalgam fillings are a "significant chronic contributor to Hg [mercury] body-burden." A
second found that children with the CPOX4 gene variant also had biomarkers of mercury-related kidney damage. The
third found neurobehavioral deficits in children who had two other gene variants that made them more susceptible to mercury.
In other words, amalgam fillings impact on your health depends on your genes, your exposure (how many fillings you have and how long you've had them) and maybe your sex. But if you're among the susceptible population and your exposure is high enough, it appears that you might suffer health consequences as a result.
These latest studies were all published between 2011 and 2013, but critics of amalgam fillings sounded the alarm long before then.
The story of U.S. regulation of amalgam fillings begins in 1976, when Congress passed the
Medical Device Amendments to the Federal Food, Drug, and Cosmetic Safety Act. The amendments required the government to place all medical devices into
one of three classifications based on risk. The riskiest items would be put into Class III, which means they would require pre-market approval by the government to verify their safety and effectiveness before they could be sold.
Years went by, and the FDA did nothing. In 2006, it released a draft white paper on amalgam filling safety and held a
two-day meeting with a panel of experts to discuss it. The experts voted down the white paper by a margin of nearly two to one.
The next year,
Moms Against Mercury and other plaintiffs filed a lawsuit against the FDA commissioner, asking the FDA (which still had not classified amalgam fillings) to remove the fillings from the market. The case was settled a year later, with the FDA promising to classify amalgam fillings by July 28, 2009.
A few days before the deadline, Moms Against Mercury and others submitted a Citizens Petition, again asking the FDA to ban amalgam fillings, or - if it was unable to do that - classify them as Class III and "seek strict proof of safety and effectiveness" before allowing them to be sold. At the very least, the group asked the FDA to place restrictions on the use of amalgam fillings in the most susceptible populations, such as pregnant women and children. Additionally, they called on the FDA to prepare an Environmental Impact Statement or an Environmental Assessment for amalgam fillings.
Days later, the FDA issued a final rule, classifying dental amalgam fillings as Class II. Class II medical devices are subject to what the FDA calls "special controls," which might include testing or warning labels, but they do not require any FDA approval before they are allowed on the market.
The petitioners almost immediately submitted a second petition, this one asking the FDA to reconsider its classification. At the time, scientists already knew the significance of the CPOX4 gene variation. The petitioners also disputed the FDA's estimation of how much mercury one was exposed to from amalgam fillings, particularly because the FDA ignored children under six and assumed that nobody got more than 10 amalgam fillings. And they felt that some of the FDA's data was out of date.
One important part of the debate is the idea of a "
reference concentration," the amount of mercury one can be exposed to without "appreciable risk of deleterious effects during a lifetime," even for sensitive individuals.
In 1995, the EPA set its
reference concentration for elemental mercury (the type of mercury in amalgam fillings) at 0.3 micrograms per cubic meter. (Jim Love, the lawyer who filed the petitions on behalf of Moms Against Mercury and others, calls their number "outdated.") California's EPA set its
reference concentration 10 times lower, at 0.03 micrograms per cubic meter.
Using 2001 to 2004 population statistics, a 2011
study estimated that, using the U.S. EPA's reference concentration, 67.2 million were getting too much mercury from their fillings.
The number of Americans absorbing an unsafe level of mercury from their fillings jumps to 122.3 million if one uses California's lower reference concentration instead.In other words, how much mercury are Americans getting from their fillings, and how much mercury equals too much? According to the FDA, Americans are not getting too much mercury from their fillings, and according to the petitioners - and the 2011 study - they are. Love, the petitioners' lawyer, feels, "It's beyond debate based on the weight of the evidence that we're getting too much mercury."
After several years without a response from the FDA, the group filed an addendum to their petition with updated science in 2013. Love is passionate about the cause.
"If we do clinical studies, are we going to find people with neurobehavioral harm?" he asks. "Are we going to find people with impaired kidney function? The answer is yes, and those studies have come out also. So when you talk to a dentist and he says there isn't any evidence, ask him if he's read our 2013 petition. I wouldn't have filed the petition if I didn't think the evidence was there."
Now, in 2013, the FDA has yet to respond to the petitions. On behalf of his clients, Love has filed a lawsuit against the FDA.
"It's a very simple lawsuit," he says. "It's under the Administrative Procedure Act. FDA is duly obligated to respond to our petition. They haven't done that. They are allowed 180 days by statute and they can ask for and receive more time."
The 180-day mark passed long ago, in 2010. The plaintiffs cannot force the FDA to ban amalgam fillings, but they can push the FDA, through the courts, to respond to their petitions. And that's what they are trying to do.
"We don't think there is an intellectually honest response that can continue to justify the ongoing use of mercury fillings," continues Love. "Our contention is that the court should and almost certainly will compel the FDA to file a response to our citizens' petitions."
He adds, "In our complaint, we spell out the fact that the largest purchaser of amalgam fillings is the US government and they supply them to the indigent, those on welfare, the US military, those on Indian reservations, and as far as we can tell from where we sit, other alternatives are not available ... One of our plaintiffs in fact is in prison. He would like his amalgam fillings removed." But since the government says the fillings are safe, the prisoner is stuck with them.
Initially, Love thought that amalgam fillings were going the way of cassette tapes and VCRs. "Lots of people get composite fillings [instead of amalgam] because they are white and they are more attractive." But, it turns out that even today, the majority of new fillings are still amalgam.
As the insurance company
Delta Dental notes, tooth-colored composite fillings are more expensive than amalgam fillings and sometimes insurance companies do not cover them, or only cover them in teeth visible in a patient's smile. The last time I had dental insurance, my insurance would have covered 90 percent of the cost of amalgam fillings but about half the cost of composite fillings. I found that out only after the dentist had placed several composite fillings in my mouth and the receptionist handed me a large bill. If I were informed of the cost difference in advance, would I have opted for mercury?
If you are worried about amalgam fillings in your mouth, you can have them removed. Kris Homme, who had hers removed in 2008, cautions that you should seek out a safe removal specialist because "a normal dentist might not use proper precautions."
. . . one can have significant amounts of the second most toxic non-radioactive metal known, mercury, in one's ≈mouth≈ is fantastically absurd.
The literature literally abounds with both studies and anecdotal accounts describing health damage subsequent to "amalgam" placement(s), and significant recovery subsequent to ≈correct≈ removal.
See Vimy, Huggins, Boyd Haley and The Int'l Academy of Oral Medicine and Toxicology. IAOMT can be seen at this [Link] on Wikipedia.
It is worth noting that the American Dental Association – merely a trade organization like the ones issuing such as mechanics' and welders' licences – owns the patents on the only amalgam compositions it will allow its members to place without censure. Not only, it has issued and maintained for years a gag order disallowing its members from discussing amalgam removal.