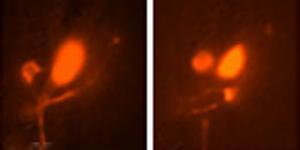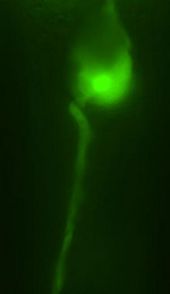
© Rockefeller UniversityWarm glow. By exploiting a cell's heat shock response, scientists use transgenes to express green fluorescent protein exclusively in amphid sheath cells, a type of nervous system cell in C. elegans. These cells do not fluoresce until scientists raise the temperature to 34 degrees Celsius.
Since our ancestors first harnessed fire, we've used heat to cook burgers, forge steel and power rockets. Now, Rockefeller University researchers are using heat for another purpose: turning genes on and off at will.
By exploiting the heat shock response, an ancient mechanism that protects cells from dangerously high temperatures, researchers have developed a new method to introduce foreign genes, called transgenes, into an organism and control when and where these transgenes are expressed. Unlike other techniques, which are labor intensive and inefficient, this new method makes controlling transgene expression as easy as turning the dial on an oven.
During heat shock, a protein called heat shock factor-1 travels from a cell's cytoplasm to the nucleus, where it binds to a specific sequence of DNA. This interaction initiates the transcription of heat shock protein, a shield that deflects excess heat from cells and protects them from damage. Since these two proteins are expressed at a specific time - when organisms experience heat shock at a specific temperature - scientists had long designed transgenes to be expressed the moment heat shock factor-1 binds to this sequence of DNA.
However, while scientists could know when this transgene was expressed, they couldn't limit its expression in specific cell types and study a particular protein's effect on them. To do so, they would have to target a single cell with a laser beam until the heat shock response kicked in for the transgene to be expressed. In
Caenorhabditis elegans, that's 34 degrees Celsius.